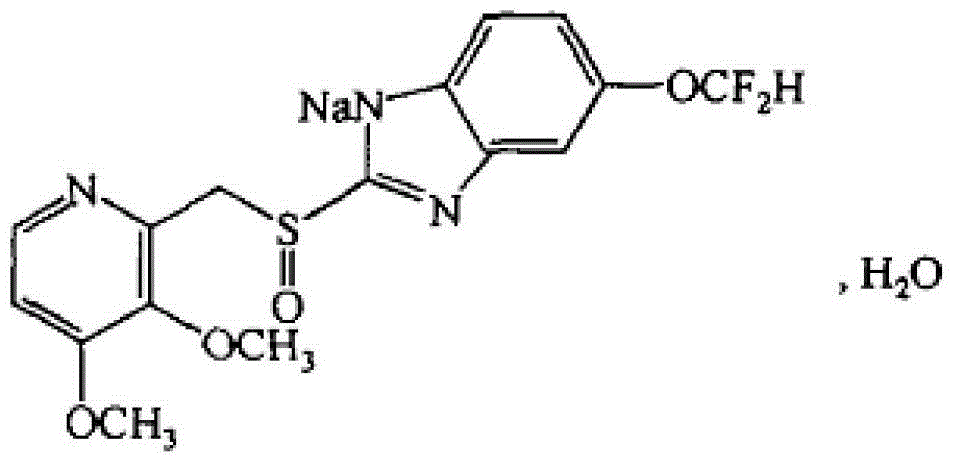
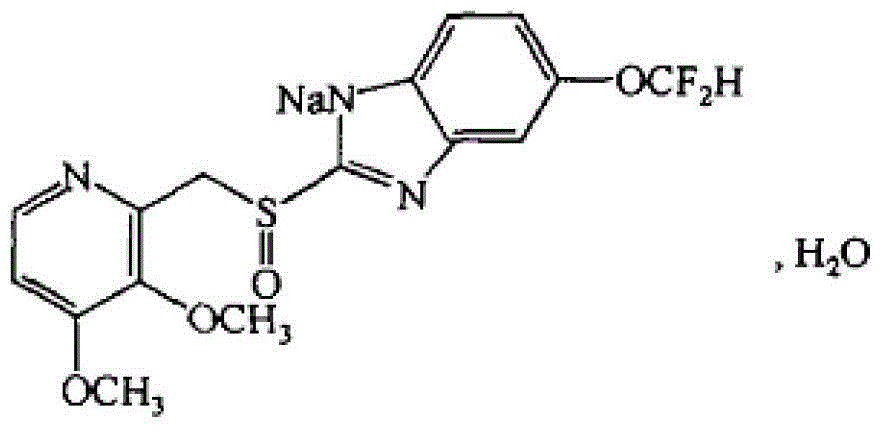
Pantoprazole sodium freeze-dried powder injection
A technology for freeze-dried powder injection and pantoprazole sodium, which is applied in the field of medicine and can solve the problems of poor water solubility, difficult preparation stability to achieve long-term stable storage and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
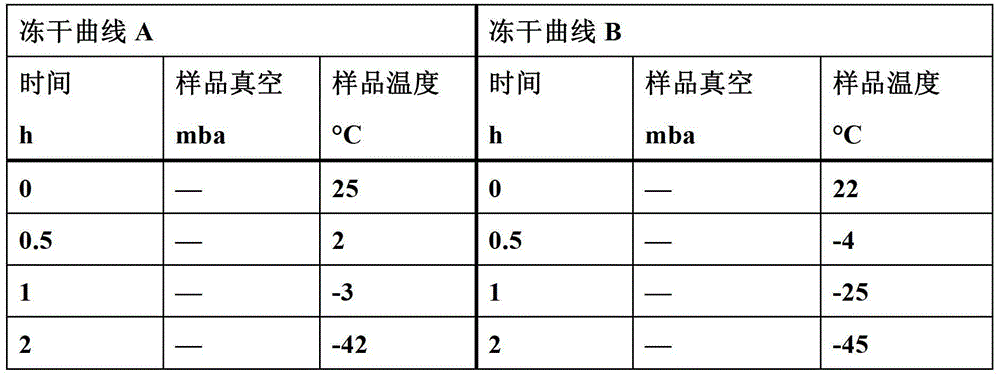
Image
Examples
preparation example 1
[0127] Preparation example 1, prepare the powder injection comprising pantoprazole sodium
[0128] formula:
[0129] Pantoprazole Sodium
40mg,
120mg,
4 mg,
5 mg,
EDTA-2Na
1.5 mg,
pH regulator
to pH10.5,
Water for Injection
Appropriate amount, add to 4ml.
[0130] Preparation:
[0131] (1) Weigh the main drug and excipients (except pH adjuster) of the prescribed amount, place them in a stainless steel bucket, add about 80% of the prescribed amount of water for injection, dissolve each component, and then add 0.1% (w / v) of activated carbon, stirred for 30 minutes, filtered and decarbonized, and added water for injection to close to the full amount of the prescription.
[0132] (2) The filtrate is sampled, and the pH value is measured, and if necessary, it is adjusted to a specified value with a pH regulator (this specified value is the value of the measured...
preparation example 2
[0137] Supplementary Preparation Example 2: Referring to the method of Preparation Example 1 above, the difference is that the citric acid is replaced with an equivalent amount of sodium citrate or the citric acid is deleted, and the numbers of the obtained powder injections are respectively Ex131 and Ex132.
[0138] Prior art product 1: prepare according to the prescription and method of preparation of CN101810588A (Chinese Patent Application No. 200910223918.1, Luo Cheng) instruction manual [0086] to [0100] paragraph embodiment 1 (the active ingredient of every bottle subpackage is pressed pantoprazole Calculated as 40mg), the powder injection number obtained is CN588A, and its preparation prescription ratio is:
[0139]
[0140] Stability study example:
[0141] Measure the chemical stability of the sample prepared in the above embodiment 1 part according to the chemical stability investigation method of this paper, after placing 4 months at 45 ° C, especially measure ...
preparation example 3
[0159] Preparation example 3, powder injection of the present invention
[0160] formula:
[0161] Pantoprazole Sodium
40mg,
120mg,
citric acid
4 mg,
5 mg,
trometamol
6mg,
EDTA-2Na
1.5 mg,
pH regulator
to pH10.5,
Water for Injection
Appropriate amount, add to 1ml.
[0162] Preparation method: refer to the method of Preparation Example 1, and the number of the obtained powder injection is marked as Ex3.
[0163] Referring to the prescription and preparation method of the above sample Ex3, the difference is that the amount of tromethamine is changed to 4mg or 8mg, and two batches of powder injections are obtained, and the numbers are respectively recorded as Ex31 and Ex32.
[0164] Referring to the prescription and preparation method of the above sample Ex3, the difference is that the amount of methionine is changed to 3mg or 7mg, and two batches of powder inj...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com