Method for purifying manganese sulfate solution
A manganese sulfate solution and manganese sulfate technology are applied in the fields of chemical industry and metallurgy, which can solve the problems of difficult recovery and disposal of heavy metal sulfides, high consumption of auxiliary materials, and large equipment investment, so as to achieve low production cost, low consumption of auxiliary materials and high production efficiency. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
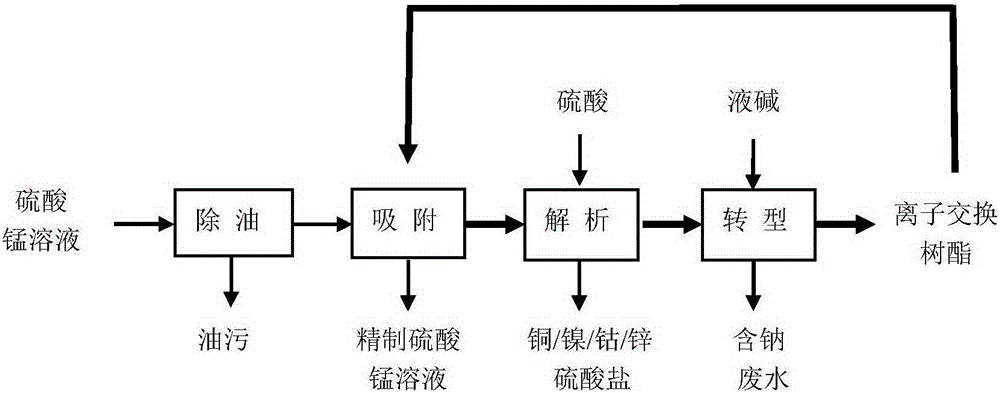
[0030] A method for purifying manganese sulfate solution, comprising the following specific steps:
[0031] (1) Degreasing: the manganese sulfate solution is degreased through microbubble air flotation, and then degreased through fibers to obtain the manganese sulfate solution after oiling;
[0032] (2) Ion exchange includes adsorption, analysis and transformation;
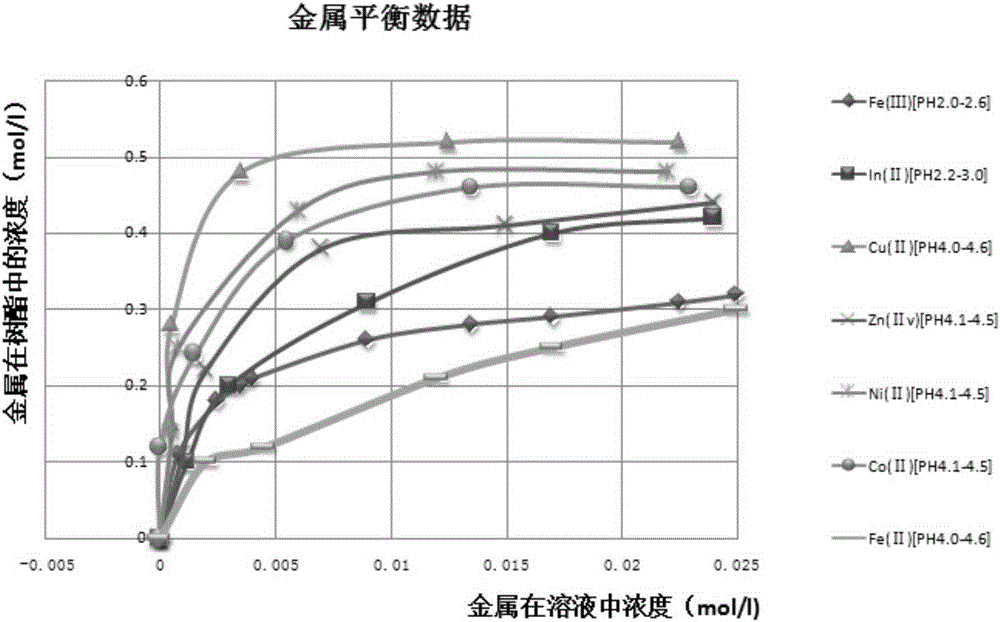
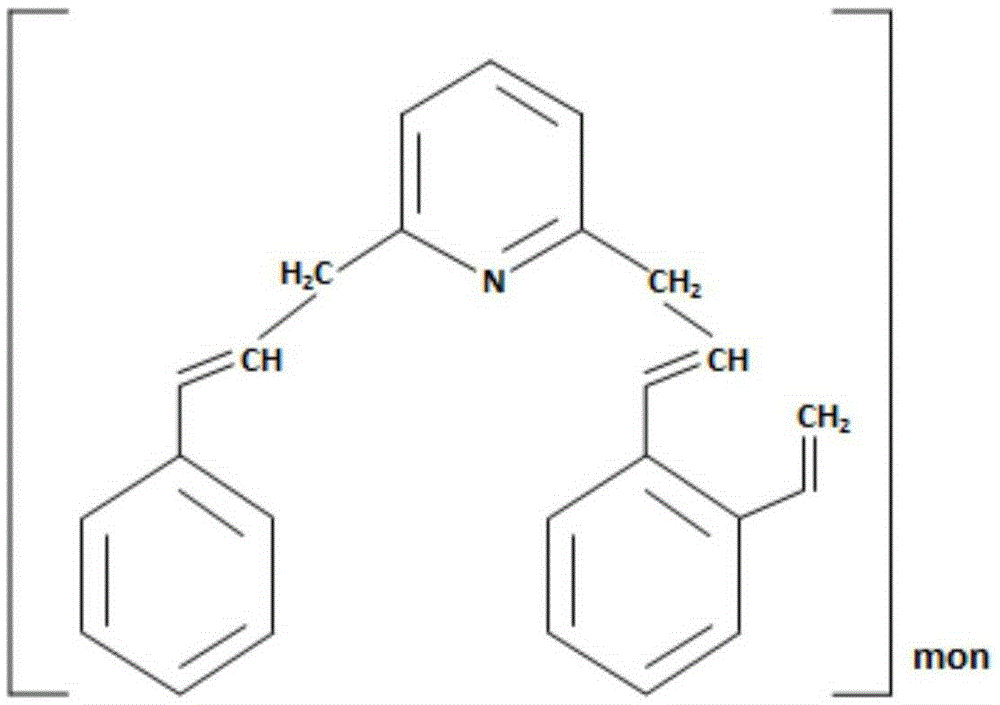
[0033] a adsorption: the manganese sulfate solution that step (1) obtains is passed through loading with lutidine group with the flow velocity of 2BV / h The ion exchange column of the chelating resin is used for adsorption, and when the content of copper, nickel, cobalt, and zinc exceeds the limit index of 2 mg / l, the adsorption operation is stopped;
[0034] b Analysis: After adsorption treatment, the resin loaded with copper, nickel, cobalt, and zinc impurities is analyzed with sulfuric acid with a mass concentration of 5% to obtain a sulfate solution containing copper, nickel, cobalt, and zinc, which is then c...
Embodiment 2
[0039] A method for purifying manganese sulfate solution, comprising the following specific steps:
[0040] (1) Degreasing: the manganese sulfate solution is degreased through microbubble air flotation, and then degreased through fibers to obtain the manganese sulfate solution after oiling;
[0041] (2) Ion exchange includes adsorption, analysis and transformation;
[0042] a adsorption: the manganese sulfate solution that step (1) obtains is passed through the manganese sulfate solution that is loaded with lutidine group with the flow velocity of 15BV / h The ion exchange column of the chelating resin is used for adsorption, and when the content of copper, nickel, cobalt, and zinc exceeds the limit index of 2 mg / l, the adsorption operation is stopped;
[0043] b Analysis: After adsorption treatment, the resin loaded with copper, nickel, cobalt, and zinc impurities is analyzed with sulfuric acid with a mass concentration of 5% to obtain a sulfate solution containing copper, ni...
Embodiment 3
[0048] A method for purifying manganese sulfate solution, comprising the following specific steps:
[0049] (1) Degreasing: the manganese sulfate solution is degreased through microbubble air flotation, and then degreased through fibers to obtain the manganese sulfate solution after oiling;
[0050] (2) Ion exchange includes adsorption, analysis and transformation;
[0051] a adsorption: the manganese sulfate solution that step (1) obtains is passed through loading with lutidine group with the flow velocity of 5BV / h The ion exchange column of the chelating resin is used for adsorption, and when the content of copper, nickel, cobalt, and zinc exceeds the limit index of 2 mg / l, the adsorption operation is stopped;
[0052] b Analysis: After adsorption treatment, the resin loaded with copper, nickel, cobalt, and zinc impurities is analyzed with sulfuric acid with a mass concentration of 10% to obtain a sulfate solution containing copper, nickel, cobalt, and zinc, which is then ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com