Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
559 results about "Cyclohexenes" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Six-carbon alicyclic hydrocarbons which contain one or more double bonds in the ring. The cyclohexadienes are not aromatic, in contrast to BENZOQUINONES which are sometimes called 2,5-cyclohexadiene-1,4-diones.
Cyclohexylene Reactive Mesogens and Their Applications
InactiveUS20100103366A1Liquid crystal compositionsOrganic chemistryLiquid crystallineReactive intermediate
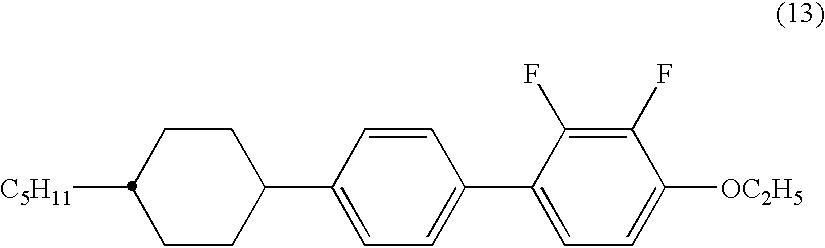
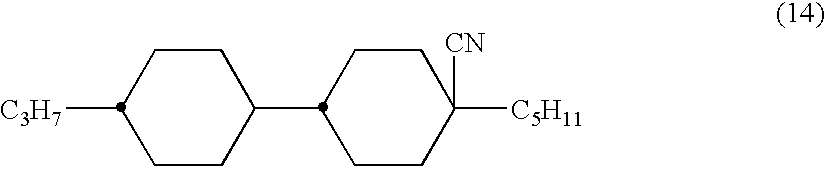
The invention relates to new cyclohexylene reactive mesogens (RM), polymers derived thereof, liquid crystal (LC) media comprising them, and the use of the compounds, polymers and liquid crystalline media in optical, electrooptical, electronic, semiconducting or luminescent components or devices, in decorative, security, cosmetic or diagnostic applications, especially in the polymer stabilised blue phase.
Owner:MERCK PATENT GMBH
Benzene selection noncrystalline catalyst with hydrogen added and containing ruthenium, boron as well as its preparing method
InactiveCN1446625AHigh catalytic efficiencyHydrocarbon by hydrogenationCatalyst activation/preparationBenzeneHydrogen
A non-crystal catalyst for preparing cyclodexene by selectively hydrogenating benzene is prepared from Ru, B meta or metal oxide modified M, and the oxide or metal hydroxide carrier L through reducing Ru ions and M in oxide form, and removing ions of impurities. Its advantage is high selectivity and hydrogenating activity.
Owner:FUDAN UNIV
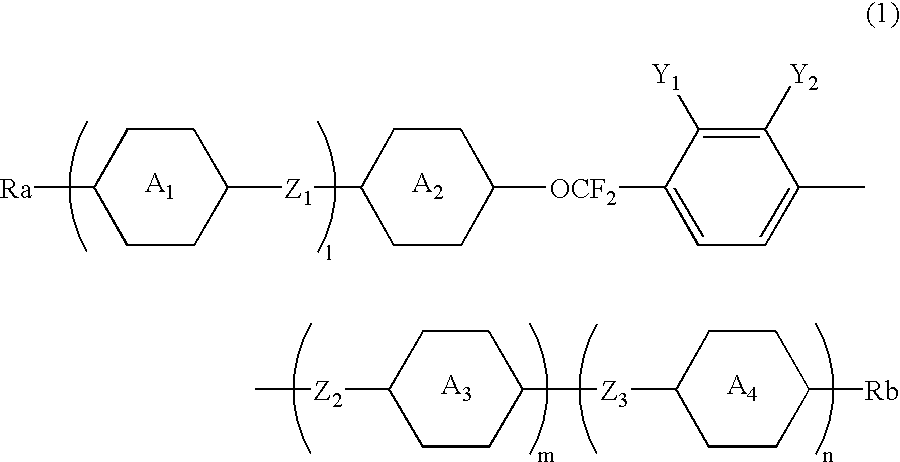
2,3-difluorophenyl derivative having negative dielectric anisotropy, liquid crystal composition, and liquid crystal display device
InactiveUS6548126B1Good compatibilityIncrease resistanceLiquid crystal compositionsOrganic chemistryLiquid crystallineCrystallography
The invention is to provide liquid crystalline compounds having a negative and extremely large dielectric anisotropy value and a small optical anisotropy value at the same time; liquid crystal compositions comprising the compound; and liquid crystal display devices fabricated by using the liquid crystal composition; the liquid crystalline compounds have 2,3-dihalogenophenylene moiety and are expressed by formula (1)wherein Ra and Rb each independently represents a linear or branched alkyl having 1 to 10 carbon, any methylene in the alkyl may be replaced by -O-, -S-, -CH=CH-, or -C=C-, but -O- is not successive, and any hydrogen in the alkyl may be replaced by halogen; rings A1 to A4 each independently represents trans-1,4-cyclohexylene, cyclohexene-1,4-diyl, pyridine-1,4-diyl, pyrimidine-2,5-diyl, or 1,4-phenylene, wherein at least one hydrogen in these rings may be replaced by halogen, and any nonadjacent methylene in cyclohexane ring may be replaced by -O-; Y1 and Y2 each independently represents F or Cl; Z1, Z2 and Z3 each independently represents a single bond, -CH2CH2-, -(CH2)4-, -COO-, -OCO-, -CH2O-, -OCH2-, -CF2O-, or -OCF2-; and l, m and n each independently is 0, 1 or 2, and the sum of l+m+n is 3 and less.
Owner:CHISSO CORP
Benzene hydrogenation catalyst as well as preparation method and application thereof
ActiveCN102600888AExcellent benzene hydrogenation catalytic performanceHigh catalytic activityMolecular sieve catalystsHydrocarbon by hydrogenationBenzeneAdjuvant
The invention relates to a benzene hydrogenation catalyst as well as a preparation method and application of the benzene hydrogenation catalyst. The catalyst consists of an active component Ru and adjuvants, wherein the active component Ru is loaded on the catalyst by a carrier; the adjuvants are one or two of La, Ce, Fe, Zn, Cu and B, and the carrier is a mesoporous molecular sieve MCM-41 modified by one or two of ZrO2, ZnO and CuO. The invention also provides a method for preparing cyclohexene and cyclohexane by benzene hydrogenation catalyzed by the catalyst. The catalyst provided by the invention can be prepared by a dipping sedimentation method or a chemical reduction method and has a higher catalytic activity and cyclohexene selectivity.
Owner:XIANGTAN UNIV
Methods, systems and catalysts for the hydration of olefins
InactiveUS20040236158A1Low compressibilityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsButenePolymer science
The present invention provides a system, method and catalyst for olefin hydration. The method includes hydrating the olefin using a base treated, sulfonated, halogenated and acid regenerated thermally stable catalyst. In several variants, the olefin hydration comprises butene hydration, propene hydration, hydration of cyclohexene, propylene hydration, pentene hydration, hexene hydration, and heptene hydration. The present invention also provides a method of making a catalyst for olefin hydration, and provides alcohols manufactured by the catalyst(s), systems and methods described herein.
Owner:COLLIN JENNIFER REICHI +1
Preparing cyclohexene catalyst and for benzene hydrogenation its manufacturing method
InactiveCN1597099AHas industrial application valueLow costHydrocarbon by hydrogenationMetal/metal-oxides/metal-hydroxide catalystsBenzeneCyclohexene
A catalyst for preparing cyclohexene from benzene by hydrogenating is prepared from metal Ru, one or two transition metals or RE elements, the hydroxide of alkali metal as precipitator, and hydrophilic indifferent oxide. It has high activity and selectivity.
Owner:ZHENGZHOU UNIV
Preparing of cyclobexene catalyst for benzene selective hydrogenation its preparation method and regulating method and regeneration method
InactiveCN1597098ANo pollution in the processLow costHydrocarbon by hydrogenationMetal/metal-oxides/metal-hydroxide catalystsBenzeneActive component
A catalyst able to be modified or regenerated by washing it with the purified water containing H2O2 and treating it by diluted acid can be used for preparing cyclohexane from benzene by selective hydrogenating. It contains an active component Ru, a RE element La or transition element Zn or Fe and a disperser, and is prepared by chemical reduction method or deposition method.
Owner:ZHENGZHOU UNIV
Process for the preparation of phenol and cyclohexanone
InactiveUS20120157718A1EfficiencyGood choiceOxygen-containing compound preparationOrganic compound preparationCyclohexanoneCyclohexene
Process for the preparation of phenol and cyclohexanone which comprises:a. the synthesis of cyclohexylbenzene by the hydro-alkylation of benzene by contact with hydrogen or the alkylation of benzene with cyclohexene using Y zeolites;b. the selective aerobic oxidation of cyclohexylbenzene to the corresponding hydroperoxide catalyzed by N-hydroxy-derivatives in the presence of polar solvents; andc. the scission of the hydroperoxide of cyclohexylbenzene to phenol and cyclohexanone by homogeneous or heterogeneous acid catalysts;characterized in that the synthesis of cyclohexylbenzene takes place in the presence of a catalytic system comprising a Y zeolite and an inorganic ligand wherein the Y zeolite has a crystalline structure with openings consisting of 12 tetrahedra and the inorganic ligand is γ-alumina, and wherein said catalytic composition is characterized by a pore volume, obtained by adding the mesoporosity and macroporosity fractions, greater than or equal to 0.7 cm3 / g, wherein at least 30% of said volume consists of pores with a diameter greater than 100 nanometers.
Owner:POLIMERI EUROPA SPA
Method for preparing cyclohexanol by using cyclohexene
InactiveCN101851151AImprove conversion rateHigh yieldOxygen-containing compound preparationOrganic compound preparationGramReaction temperature
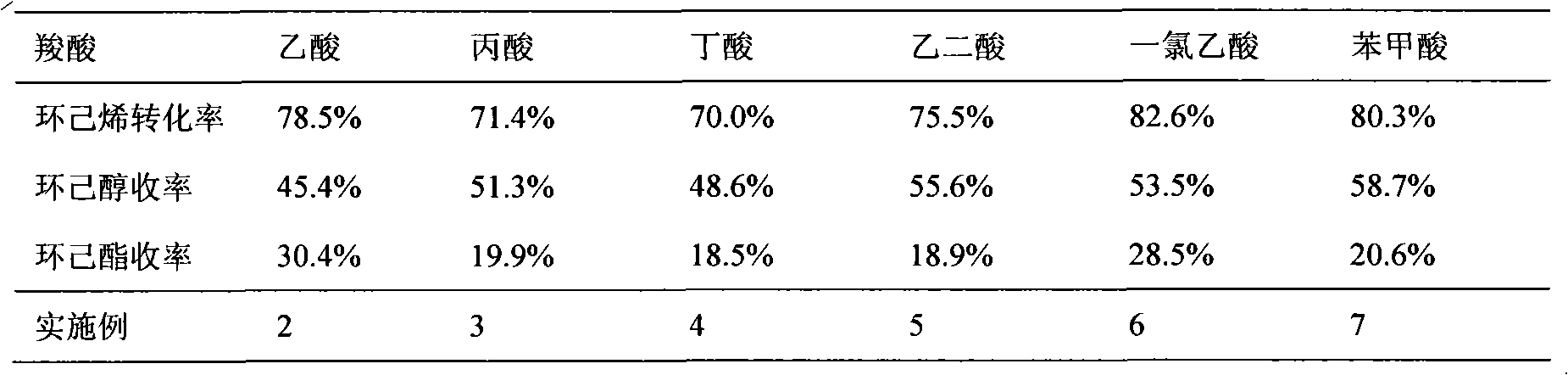
The invention discloses a method for preparing cyclohexanol by using cyclohexene. The method comprises the following steps of: (1) adding cyclohexene, carboxylic acid and catalyst into a reactor, heating the mixture to 100 to 150 DEG C and keeping the temperature to perform a reaction for 0.5 to 8 hours, wherein the mole ratio of the cyclohexene to the carboxylic acid is 1: 0.5-10, the use amountof the catalyst is 0.05 to 0.5 grams of catalyst per millimeter carboxylic acid, and the system pressure is the system self-generated pressure at the reaction temperature; and (2) after step (1) finishes, adding de-ionized water into the reactor, heating the mixed solution to 150 to 200 DEG C and continuously performing a reaction for 0.5 to 8 hours to obtain the cyclohexanol, wherein the volume ratio of the added de-ionized water to the original cyclohexene is 1 to 20: 1. In the invention, a simple intermittent reactor is adopted and the two reaction steps are completed in one reactor, so the equipment investment is small, the operation is simple and the cost is low. Besides, the catalyst used is easy to separate and can be repeatedly used, so the production cost is reduced.
Owner:HEBEI UNIV OF TECH
Catalyst for use in ternary polymerization of norbornene, maleic anhydride and cyclohexene and ternary polymerization method
The invention relates to a catalyst for use in ternary polymerization of norbornene, maleic anhydride and cyclohexene and a ternary polymerization method. The catalyst is characterized by being prepared by using a method comprising the following steps of: dissolving ferric trichloride, a ligand and an alkyl metal compound into a first solvent, putting into a dry single-mouth glass bottle in an inert atmosphere, sealing a bottleneck with a latex tube, preserving heat in a water bath of 50 DEG C for 20-30 minutes, and cooling to obtain an iron complex catalyst; adding norbornene, maleic anhydride and cyclohexene into a dry single-mouth glass bottle in an inert atmosphere, adding a second solvent into the single-mouth glass bottle, dissolving, and sealing the bottleneck; adding the iron complex catalyst into the single-mouth glass bottle, and reacting at the constant temperature of 40-100 DEG C for 2-3 hours; and precipitating an obtained reaction product, washing and drying to obtain a norbornene-maleic anhydride-cyclohexene terpolymer. The catalyst provided by the invention can be used for catalyzing polymerization under the normal pressure at a low temperature, and the copolymer yield is high.
Owner:NINGBO UNIVERSITY OF TECHNOLOGY
Heptamethine cyanine active fluorescent probe and preparation method and application thereof
InactiveCN108033907AImprove stabilityReduce background fluorescenceOrganic chemistryFluorescence/phosphorescenceChemical reactionStructural formula
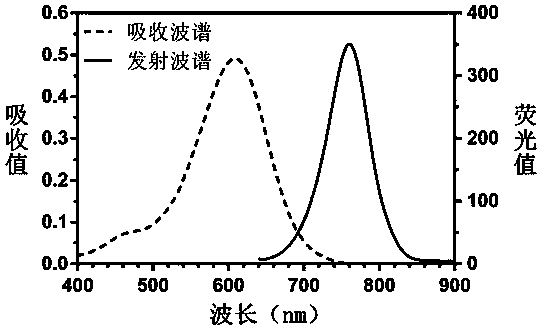
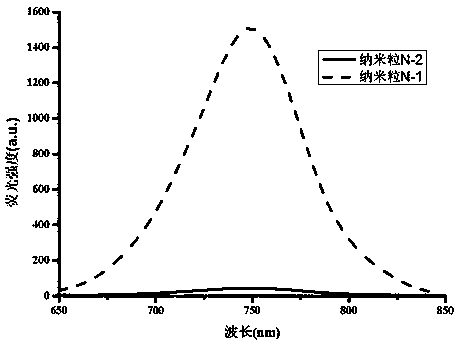
The invention relates to a heptamethine cyanine active fluorescent probe and a preparation method and application thereof. The structural formula of the heptamethine cyanine active fluorescent probe is as shown in the specification, wherein X=II-IX; each of R1 and R2 is (CH2)mCH3, (CH2)nOH, (CH2CH2O)pCH3 and CH2C6H5; each of R3 and R4 is H, SO3H, SO3Na and SO3K; each of a, b, c, d, e, f and g is 2-8; each of n, m and p is 1-10. The heptamethine cyanine active fluorescent probe has the advantages that the heptamethine cyanine active fluorescent probe is based on near-infrared long-wave heptamethine cyanine dye, indoline is selected as the aroma parent nucleus to increase fluorescence intensity, and methenyl chain intermediate cyclohexene rigid bridging enhances stability; nitrogen derivatives with chemical reactivity sites are used to perform nucleophilic substitution on the meso-position of the heptamethine cyanine parent dye, and accordingly Stokes shift and active chemical groups areincreased greatly to facilitate the fluorescent labeling of various substances; the fluorescent probe is of a symmetrical structure, preparation and purification processes are simplified, and cost islowered favorably; the probe can be used as the fluorescent labeling probe of biological molecules such as high-sensitivity protein, sugar and DNA and nano carriers to perform cell or living-body horizontal fluorescence imaging, and the like.
Owner:INST OF BIOMEDICAL ENG CHINESE ACAD OF MEDICAL SCI
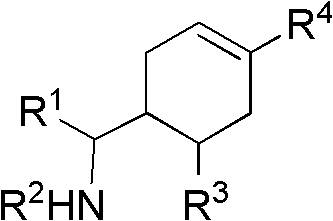
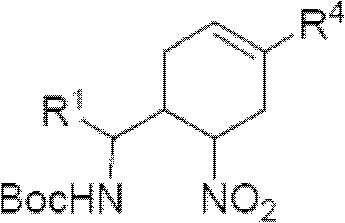
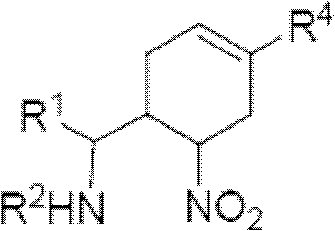
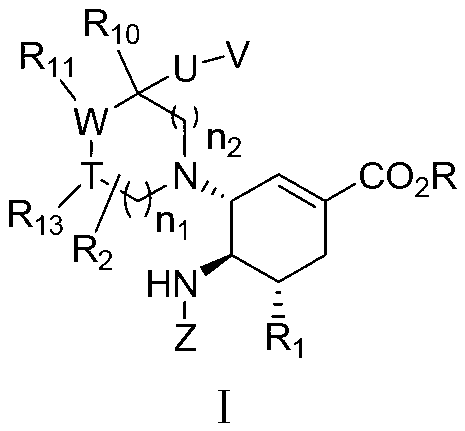
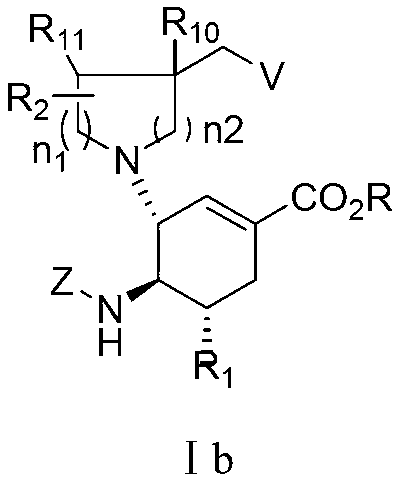
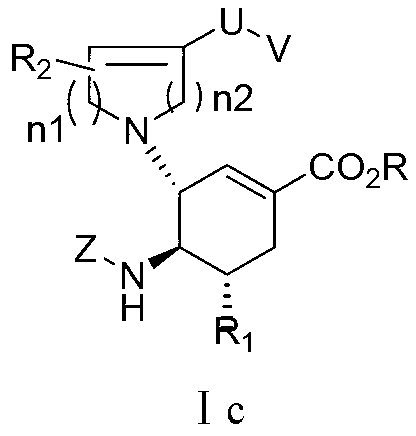
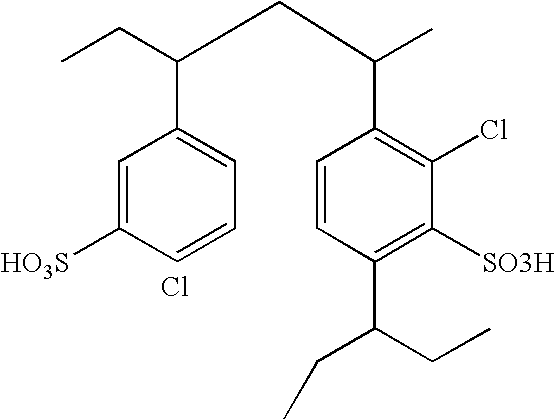
3-Pyrrolo[b]cyclohexylene-2-dihydroindolinone derivatives and uses thereof
3-pyrrolo[b]cyclohexylene-2-dihydro-indolinone derivatives of formula (I) or their pharmaceutically acceptable salts and uses thereof. The intermediates of formula (II) for preparing the above compounds. The bioassay shows that the above compounds and their pharmaceutically acceptable salts can modulate the activity of protein kinases (PKs), inhibit the activity of tyrosine kinases (PTKs) and inhibit many kinds of tumor cells as well as.
Owner:JIANGSU SIMCERE PHARMA
Integral catalyst for producing cyclohexene with benzene hydrogenation and method for producing the same
InactiveCN101269326AEasy to prepareLow costHydrocarbon by hydrogenationMetal/metal-oxides/metal-hydroxide catalystsActive componentLanthanum compounds
The invention relates to an integral catalyst used in making cyclohexene from benzene hydrogenation, taking a cordierite ceramic honeycomb or a metal honeycomb as a carrier, being coated with an additive accounting for 1-50wt percent of a honeycomb carrier in weight and an active component accounting for 0.05-10wt percent of a cellular carrier in weight; an accessory ingredient is one or more than one among Al2O3, SiO2, TiO2, ZrO2, La2O3, Fe2O3, ZnO, Cr2O3, GaO, CuO, BaO, CaO; the active component is precious metal such as Ru, Pt, Pd, or Rh, or the mixture of the precious metal and the additive. The integral honeycomb catalyst of the invention has a simple preparation method and the raw material is wide and easy to get. The active component of the integral catalyst of the invention is not so easy to lose, thereby reducing the amount of the precious metal, besides, the higher yield of cyclohexene and the selectivity can be obtained.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Liquid-phase and vapor-phase dehydration of organic / water solutions
InactiveUS20120190091A1Satisfactory operationHigh selectivitySemi-permeable membranesOxygen-containing compound preparationAlcoholGas phase
Disclosed herein are processes for removing water from organic compounds, especially polar compounds such as alcohols. The processes include a membrane-based dehydration step, using a membrane that has a dioxole-based polymer selective layer or the like and a hydrophilic selective layer, and can operate even when the stream to be treated has a high water content, such as 10 wt % or more. The processes are particularly useful for dehydrating ethanol.
Owner:MEMBRANE TECH & RES
ZSM-5 structure zeolite, preparation and use thereof
InactiveCN1257840CTroubleshooting FiltrationImprove conversion rateMolecular sieve catalystsPentasil aluminosilicate zeoliteHydration reactionPtru catalyst
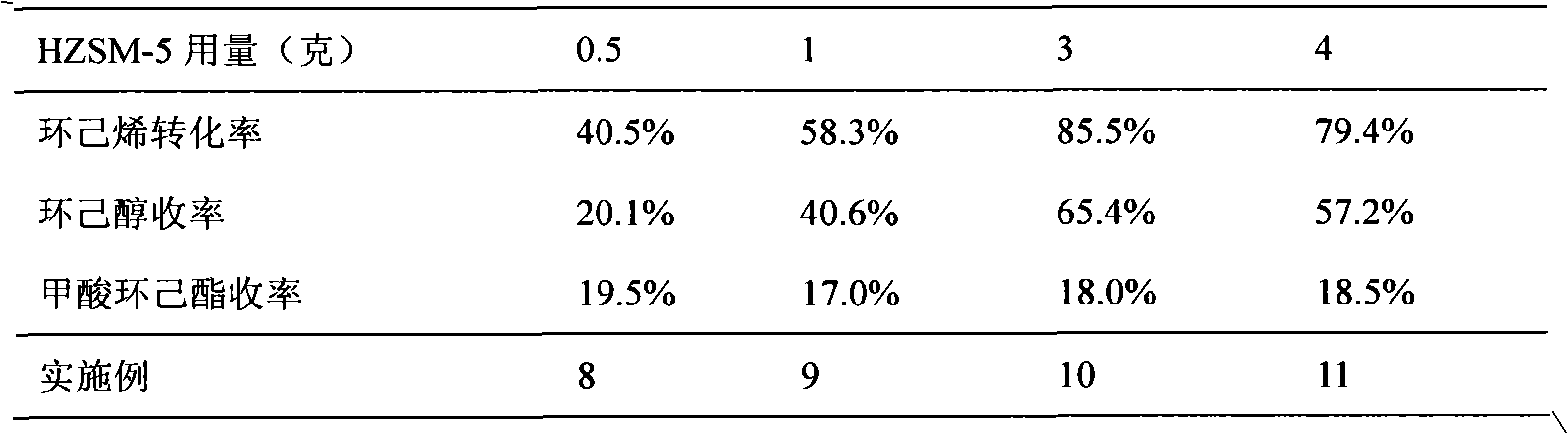
A ZSM-5 structured zeolite, the molar ratio of silicon oxide to aluminum oxide is 15-200, the primary particle diameter is 0.5-10 μm, and the mesopore surface area with a pore diameter of 2-50 nm measured by BET low-temperature nitrogen adsorption method is 25- 250m 2 / g. The zeolite is prepared from conventionally produced zeolite with larger crystal grains as raw material, treated with alkaline aqueous solution, ammonium ion exchange, dried and roasted. When the zeolite is used as a catalyst for the hydration of cyclohexene to prepare cyclohexanol, a good conversion rate of cyclohexene can be obtained, and it solves the difficulty in separating the catalyst from the liquid phase after the hydration reaction in the prior art and the fine crystal grains during the zeolite synthesis. resulting in difficult filtering problems.
Owner:CHINA PETROLEUM & CHEM CORP +1
Catalyst for ternary polymerization of norbornene, derivative of norbornene, tetrafluoroethylene and cyclohexene and method of ternary polymerization
The invention relates to a catalyst for ternary polymerization of norbornene, a derivative of the norbornene, tetrafluoroethylene and cyclohexene and a method of the ternary polymerization. The preparation method of the catalyst comprises steps of dissolving ruthenium trichloride, ligand and an alkyl metal compound to a first solvent in a dry single-opening glass bottle in which inert gas is filled, sealing the bottle opening by using an emulsion tube, keeping the bottle at the constant temperature for 20-30 minutes in the water bath of 50 DEC C, cooling and obtaining a ruthenium complex catalyst. Compared with the prior art, the catalyst is the novel ruthenium complex catalyst, the catalyst can produce catalytic activity under certain pressure, at the temperature from 20 DEG C to 120 DEG C and in the nitrogen protection system, the norbornene, the tetrafluoroethylene and the cyclohexene are catalyzed and are subjected to the ternary polymerization, and products are easy to wash and separate after being soaked by methanol. The yield of copolymer is high, the reaction temperature is low, the catalyst is easy to obtain and the catalyst efficiency is high.
Owner:NINGBO UNIVERSITY OF TECHNOLOGY
Epoxy resin composition excellent in weather resistance and fiber-reinforced composite materials
InactiveUS6902811B2Good weather resistanceHollow inflatable ballsHollow non-inflatable ballsEpoxyPolymer science
The present invention relates to an epoxy resin composition comprising at least (A) an epoxy resin having a cycloalkane ring or cyclohexene ring but having neither aromatic ring nor aminic nitrogen atom, (B) a carboxylic anhydride having no aromatic ring, and (C) an ultraviolet absorbent, wherein a content of component (A) is from 70 to 100 percents by weight based on the total weight of epoxy resins, and a content of component (B) is from 70 to 100 percents by weight based on the total weight of carboxylic anhydrides. By this constitution, the present invention can provide an epoxy resin composition whose cured product excels in mechanical properties and weatherability, and a fiber reinforced composite material having excellent mechanical properties and weatherability.
Owner:TORAY IND INC
Method for preparing sulfonic acid group-modified mesoporous material-loaded heteropolyacid catalyst and application thereof during esterification reaction
ActiveCN105854942AImprove hydrophobic propertiesEasy to prepareOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsFormic acidOxidizing agent
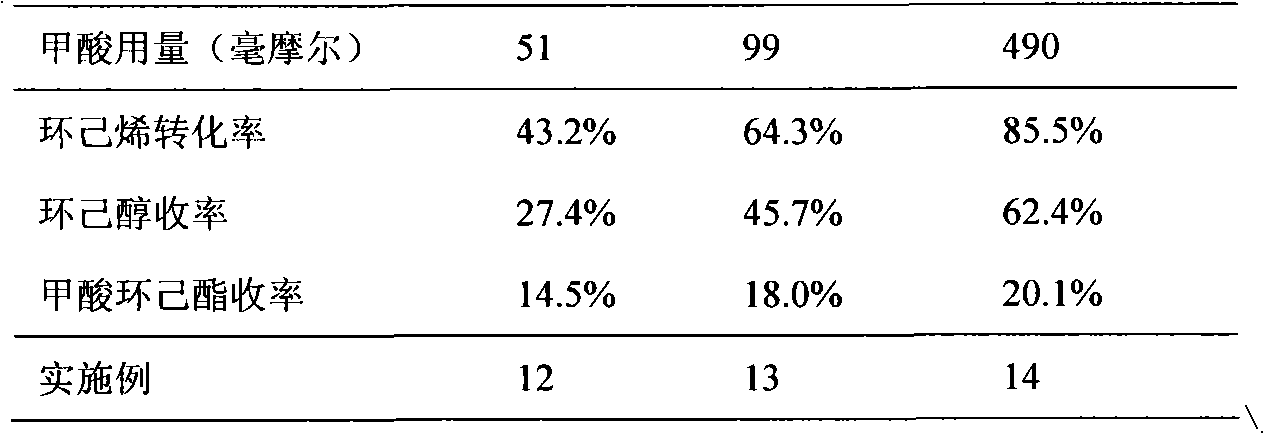
The present invention discloses a method for preparing a sulfonic acid group-modified mesoporous material-loaded heteropolyacid catalyst and the application thereof during the esterification reaction. 3-mercapto propyl trimethoxy silane is adopted as an organic silicon source, and tetraethoxysilane is adopted as an inorganic silicon source. A tri-block copolymer is adopted as a template agent, and H2O2 solution is adopted as an oxidant. Phosphotungstic acid is added as an active ingredient and the sulfonic acid group-modified mesoporous material-loaded heteropolyacid catalyst is obtained through the one-step hydro-thermal synthesis process based on the copolycondensation method in the acidic condition. According to the technical scheme of the invention, the catalyst has the advantage of strong acidity, and the specific surface area thereof is 300-600 m2 / g. The pore volume of the catalyst is 0.4-1.4 cm3 / g, and the pore diameter of the catalyst is 3-7 nm. When the catalyst is used for catalyzing the esterification reaction of cyclohexene and formic acid, the catalyst exhibits good catalytic activity. The conversion rate of cyclohexene can reach 87% and the selectivity of cyclohexyl formate is up to 99%. Moreover, even after the catalyst is recycled, the catalyst is still good in activity. Therefore, the catalyst can be recycled and used repeatedly, and has a considerable industrial application prospect.
Owner:XIANGTAN UNIV
Method for preparing cyclopentene/cyclohexene-1-boronic acid pinacol ester
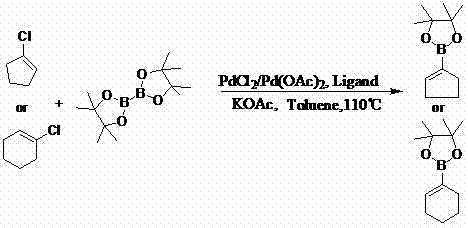
InactiveCN103044469AReduce dosageLow costGroup 3/13 element organic compoundsCyclopentenePtru catalyst
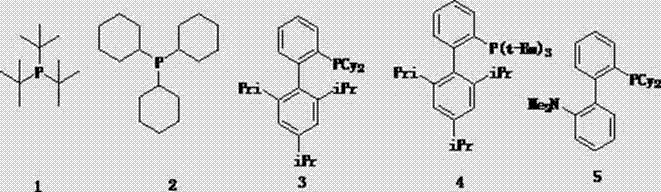
The invention discloses a method for preparing cyclopentene / cyclohexene-1-boronic acid pinacol ester. The method comprises the step of carrying out coupling reaction on 1-chlorine-cyclopentene / cyclohexene serving as a raw material in an organic solvent A in the presence of monophosphate ligand and a palladium catalyst based on potassium acetate as alkali so as to prepare the cyclopentene / cyclohexene-1-boronic acid pinacol ester. The method is characterized in that the physicochemical reaction at the n-butyllithium and ultralow temperature condition in the literature is avoided, and the palladium catalyst is adopted for catalyzing, so that the amount of the used catalyst is reduced, and the cyclopentene / cyclohexene-1-boronic acid pinacol ester can be prepared in relatively high yield at the acceptable temperature; the boronizing agent n-butyllithium / isopropoxy boronic acid pinacol ester system is replaced by the boronizing agent palladium catalyst / bis(pinacolato)diboron system, and therefore, the cost of the raw materials is greatly reduced, the reaction condition is relatively easy to realize, amplification is easy, and the technical operation is simpler and more convenient.
Owner:DALIAN NETCHEM CHIRAL TECH
Cyclohexene compound having influenza virus neuraminidase inhibition activity, preparation method and application
InactiveCN102964267AHas neuraminidase inhibitory activityOvercoming drug resistanceCarbamic acid derivatives preparationCarboxylic acid nitrile preparationCyclohexeneEnantiomer
The invention provides a compound expressed by a general formula I, or its pharmaceutically acceptable salt, and an analyzed enantiomer and a purified diastereomer, wherein R<1>is H or C1-12 alkyl groups; R<2> is H, -C(O)R<1a>, -C(O)OR<1a> or -S(O)2R<1b>, wherein R<1a> is H or C1-6 alkyl groups, R<1b> is selected from H or C1-6 alkyl groups halogenated hydrocarbon, phenyl or aryl group; R<3> is selected from C1-4 alkyl groups substituted amino, halogen, hydroxyl, sulfydryl, guanidyl, nitryl or cyano group; and R<4> is -(CH2)nCO2H, -(CH2)nP(O)(OH)2, -(CH2)nCO2R<1a> and -(CH2)nP(O)(OR<1a>)2, wherein R<1a> is H or C1-6 alkyl groups, n is an integer between 0 and 4, or a salt of the above groups. The invention also provides a preparation method of the cyclohexene compound expressed by the general formula I, and an application of the cyclohexene compound taken as an influenza virus neuraminidase inhibitor for preparing the medicines to prevent or treat the influenza diseases.
Owner:SUN YAT SEN UNIV
Cyclohexene derivative or its pharmaceutically acceptable salt and application thereof
ActiveCN103224464AStrong inhibitory activityHigh activityOrganic chemistryAntiviralsCyclohexeneOseltamivir resistant
Belonging to the field of pharmaceutical chemistry, the invention discloses a cyclohexene derivative shown as formula I or its pharmaceutically acceptable salt. The cyclohexene derivative or its pharmaceutically acceptable salt has good inhibitory activity on influenza virus neuraminidase, and especially has high activity on the neuraminidase of oseltamivir resistant influenza virus strains.
Owner:GUANGZHOU HENOVCOM BIOSCI CO LTD
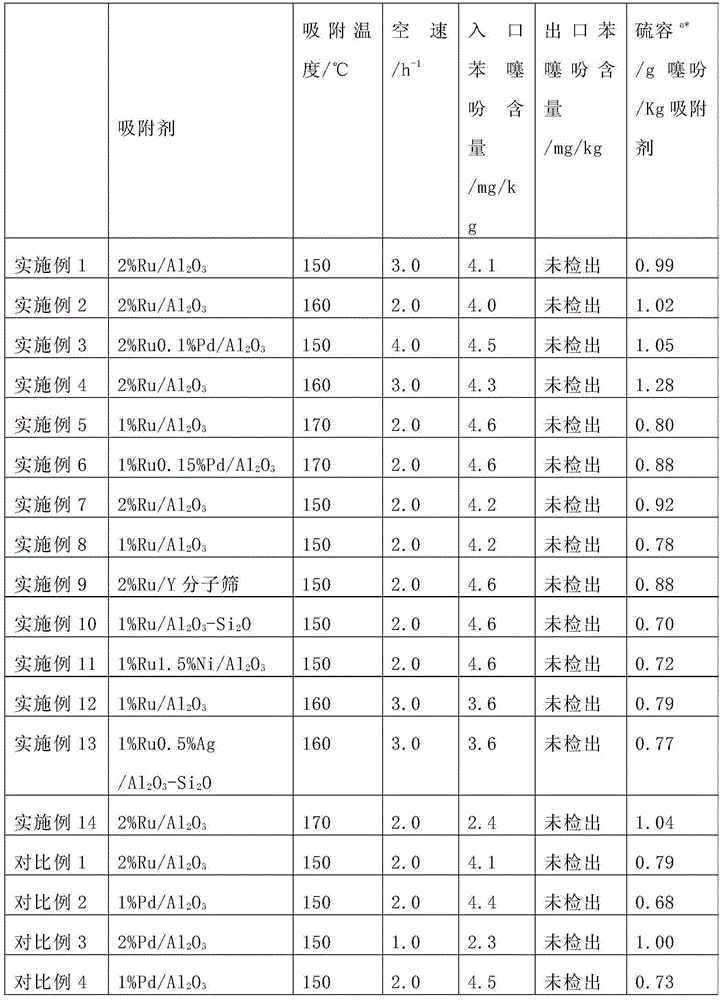
Ruthenium-based adsorbent for deeply removing thiophene of benzene as well as preparation method and application of ruthenium-based adsorbent
ActiveCN104307464AObvious price advantageSimple preparation processOther chemical processesAdsorption purification/separationBenzeneMolecular sieve
The invention provides a ruthenium-based adsorbent for deeply removing thiophene of benzene as well as a preparation method and application of the ruthenium-based adsorbent. The ruthenium-based adsorbent comprises metal ruthenium loaded on a carrier, wherein the carrier is selected from aluminum oxide, silicon oxide, titanium oxide and a molecular sieve, and a mixture of any two or more thereof. The preparation method comprises the following steps: adopting a molded porous grain-shaped carrier; carrying out modification treatment on the carrier in advance and loading pre-blended ruthenium-containing compound slurry by an immersion or spraying method; adding an inorganic alkali solution to carry out sedimentation and transformation; and carrying out liquid-phase reduction and / or gas-phase reduction to obtain the ruthenium-based adsorbent. The benzene, which is treated by the novel ruthenium-based adsorbent, can meet the requirements on content and quality of thiophene in the raw material benzene in a process of preparing cyclohexene through hydrogenating the benzene. Compared with the prior art, the ruthenium-based adsorbent has high thiophene removal precision, high sulfur capacity, low price and simple production process and equipment; and industrialized production is easy to realize and the ruthenium-based adsorbent has good application prospect and great economic benefits.
Owner:SHANGHAI XUNKAI NEW MATERIAL TECH
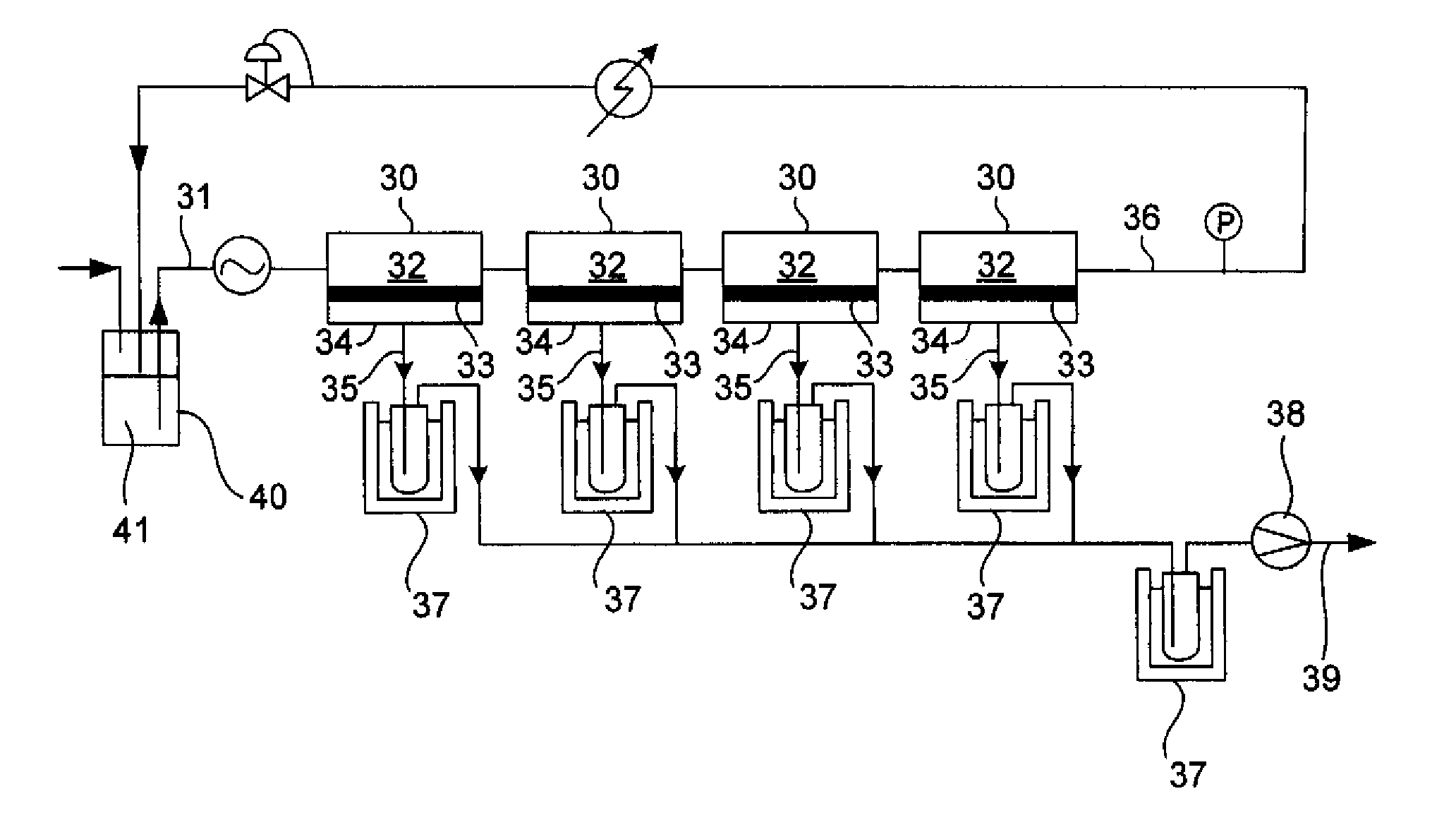
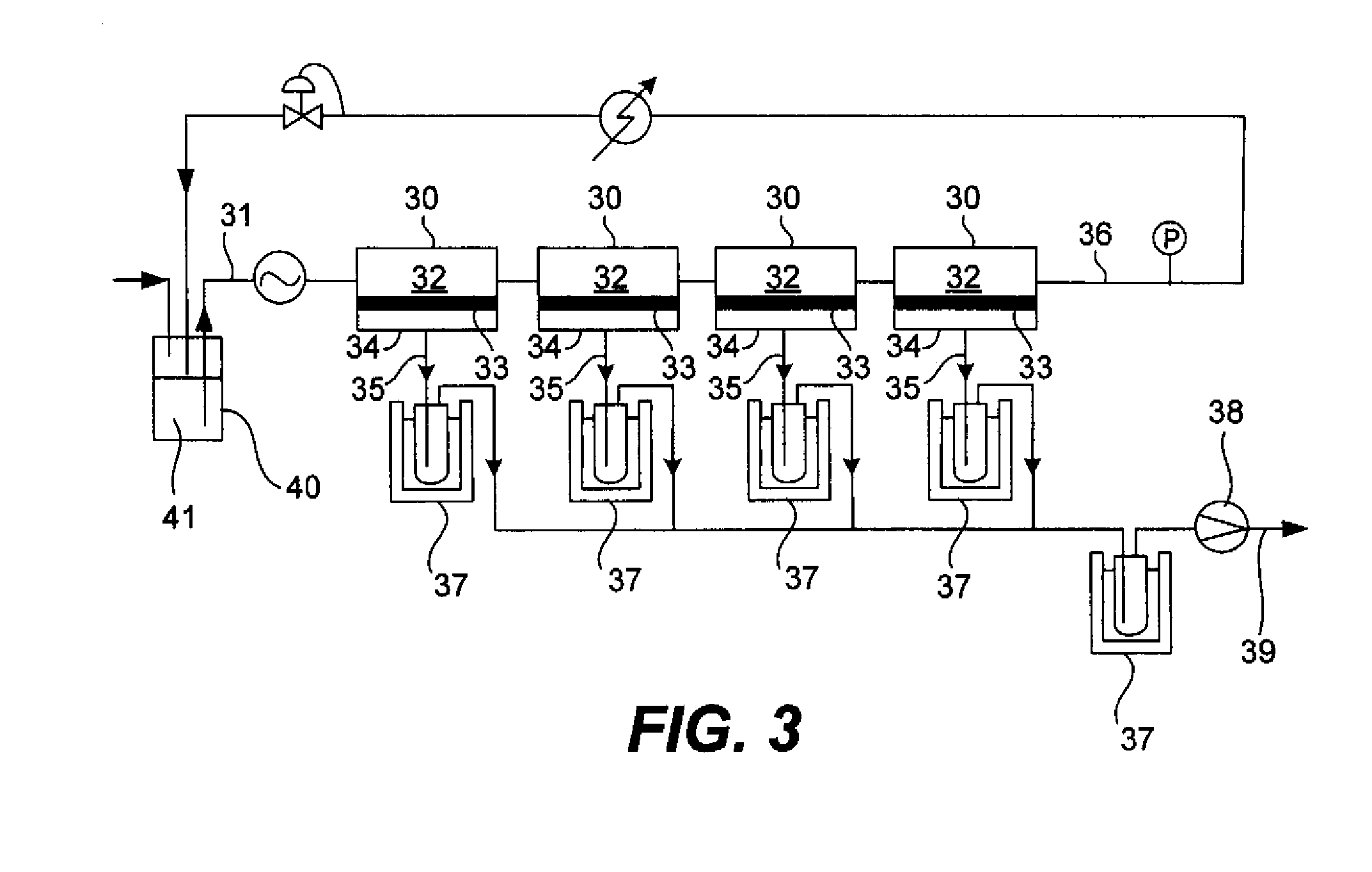
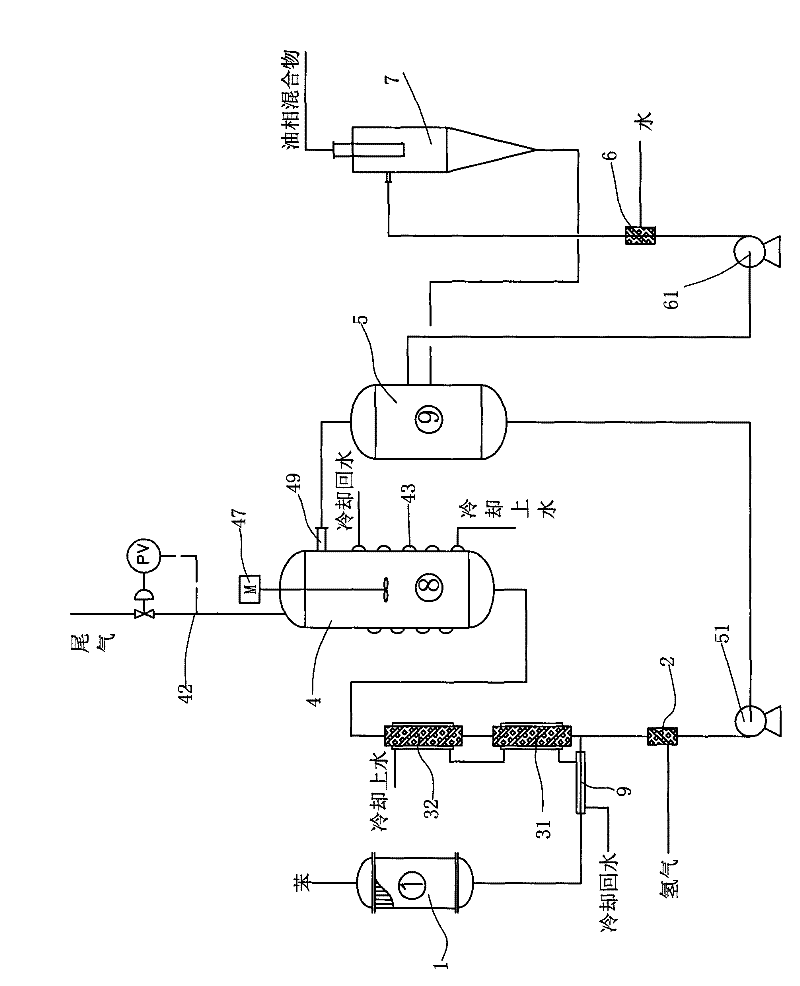
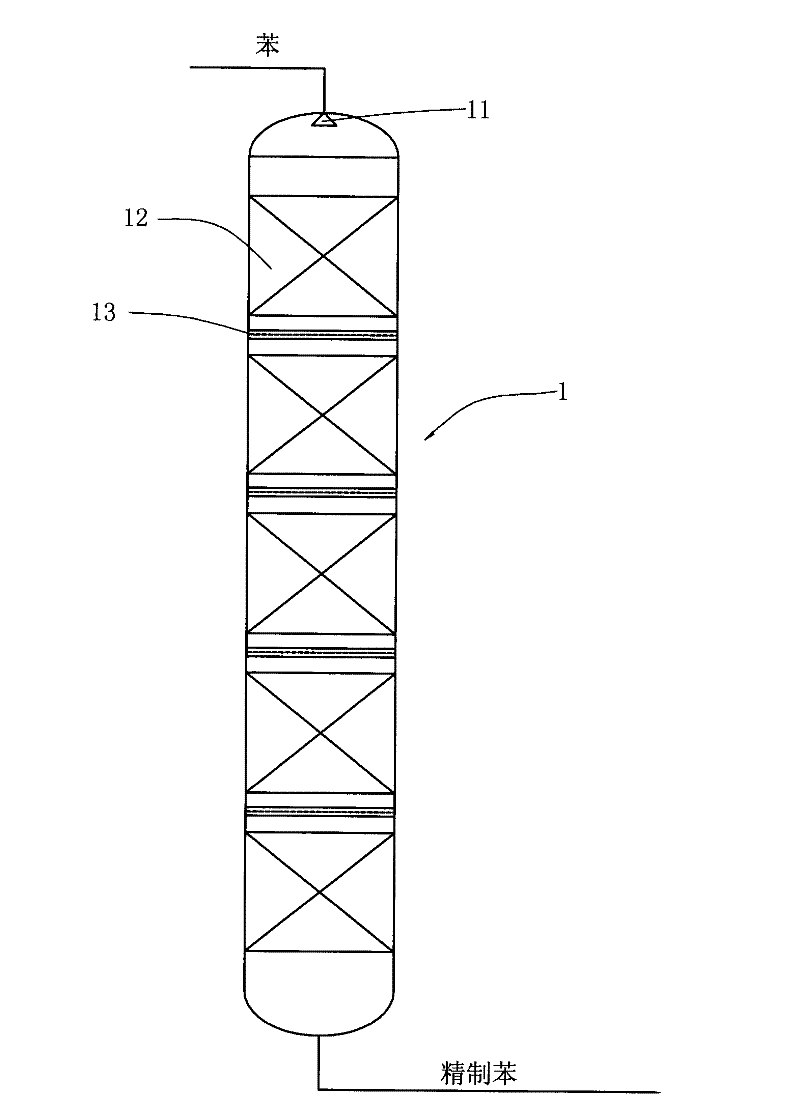
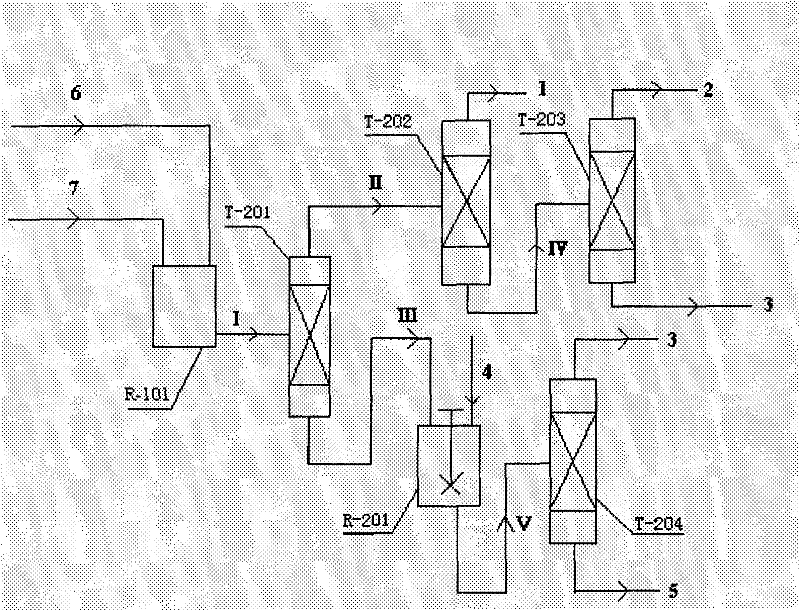
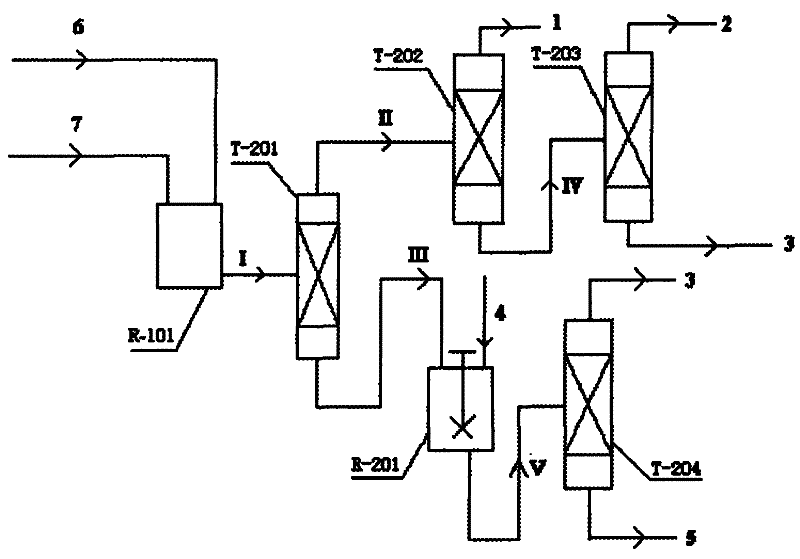
Reaction device and process for preparing cyclohexene by selectively hydrogenating benzene
ActiveCN102241558AImprove continuityIncrease production capacityHydrocarbon by hydrogenationBenzeneHydrogen
The invention relates to a reaction device and process for preparing cyclohexene by selectively hydrogenating benzene. The reaction device comprises a benzene refiner, a gas-liquid mixer, a static mixed reactor and a separation tank, wherein the benzene refiner is used for refining raw material benzene; the gas-liquid mixer is used for mixing hydrogen with catalyst slurry; the static mixed reactor is taken as a main place of selective hydrogenation; and the separation tank is used for separating a reaction product from a catalyst, wherein the reaction product and the catalyst are conveyed by using the static mixer reactor. A process method for finishing a reaction comprises the following steps of: feeding the raw material benzene into the benzene refiner for refining, and exchanging heat to 100-130 DEG C; feeding the catalyst slurry and the hydrogen into the gas-liquid mixer with a certain pressure according to a certain proportion simultaneously for fully mixing; feeding the refined benzene and the catalyst slurry obtained by mixing into the static mixed reactor; controlling certain reaction temperature, reaction pressure and liquid flow rate; making the materials react in the static mixed reactor to obtain a cyclohexene-containing mixture; and feeding the cyclohexene-containing mixture into the separation tank for separating. Compared with the prior art, the process has the advantages of stable and easily-controlled reaction and high benzene transformation rate and cyclohexene selectivity.
Owner:SEDIN NINGBO ENG +1
Technique for producing cyclohexenol through benzene plus hydrogen, catalyst applied and preparation method
A process for preparing cyclohexene from benzene by hydrogenation includes creating reaction, pretreating and hydrogenating reaction of benzene. Its Ru-Fe-B / ZrO2 catalyst with high activity and selectivity and its preparing process are also disclosed.
Owner:ZHENGZHOU UNIV
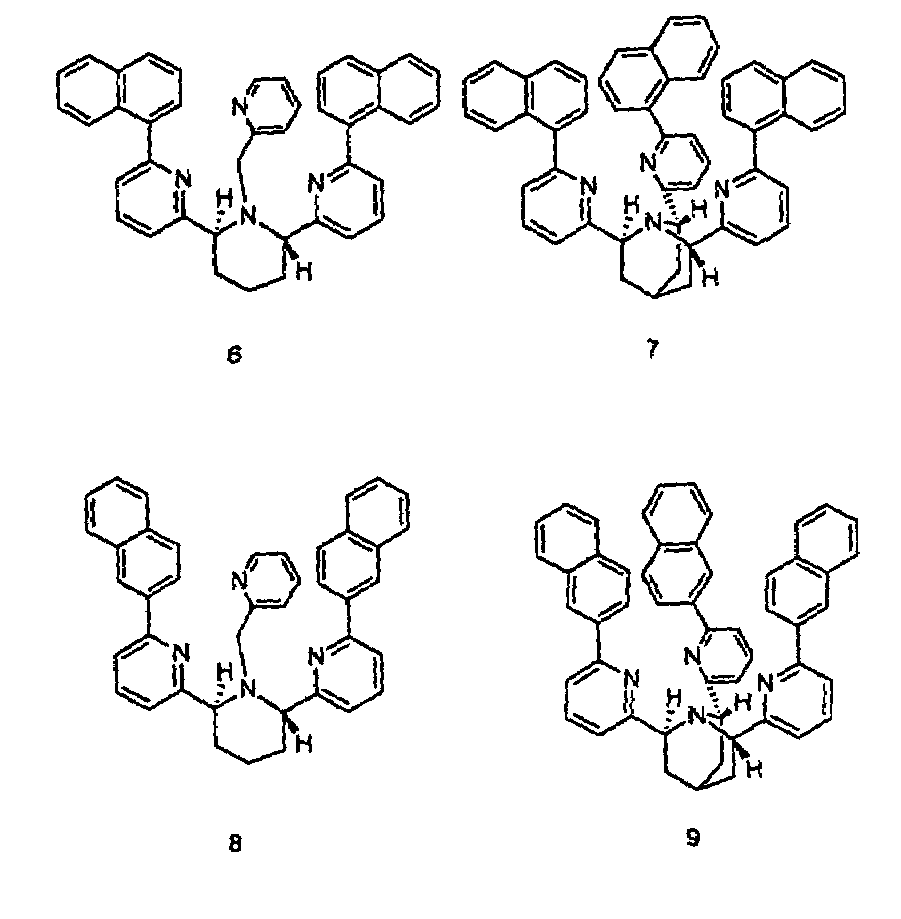
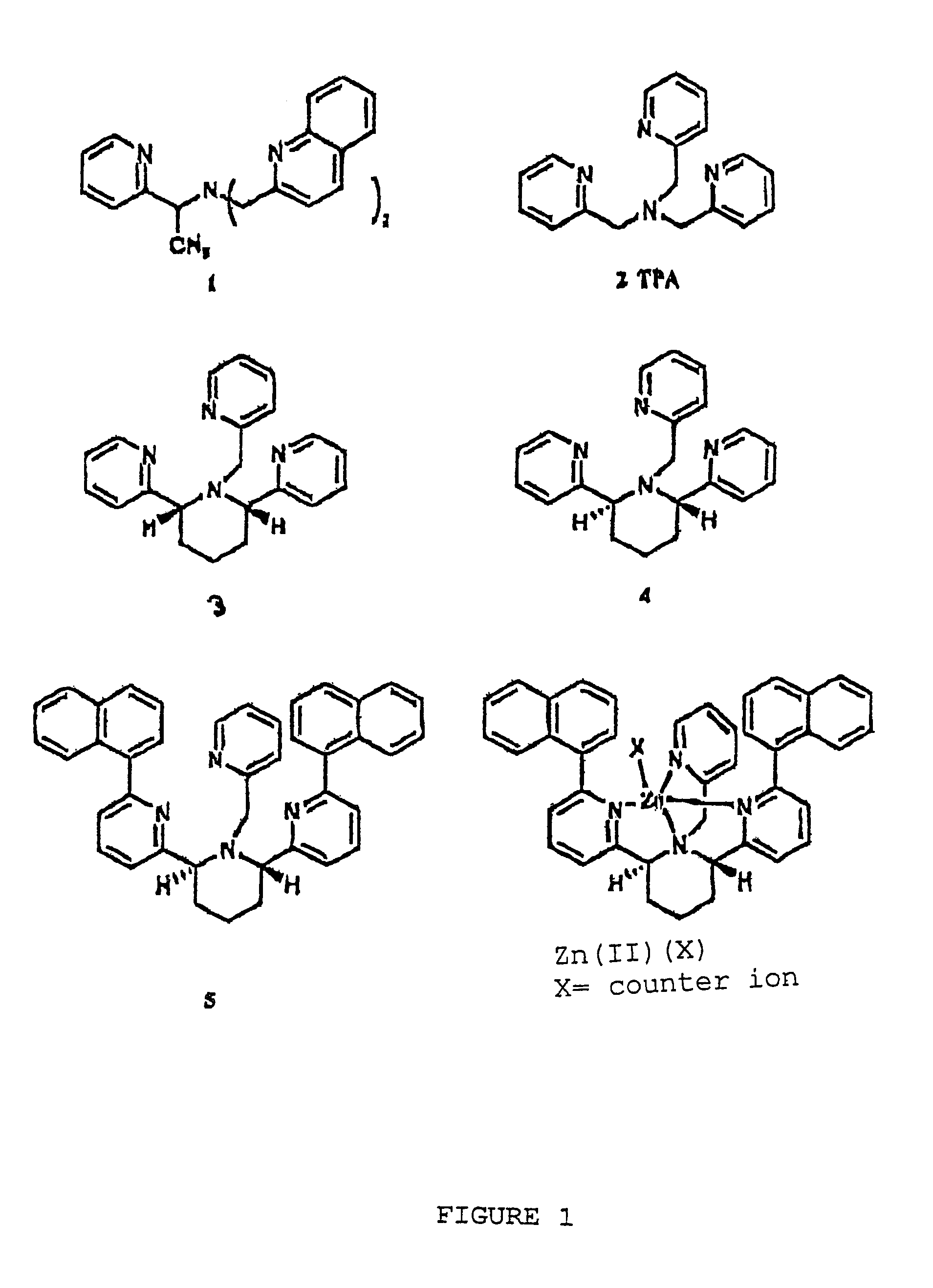
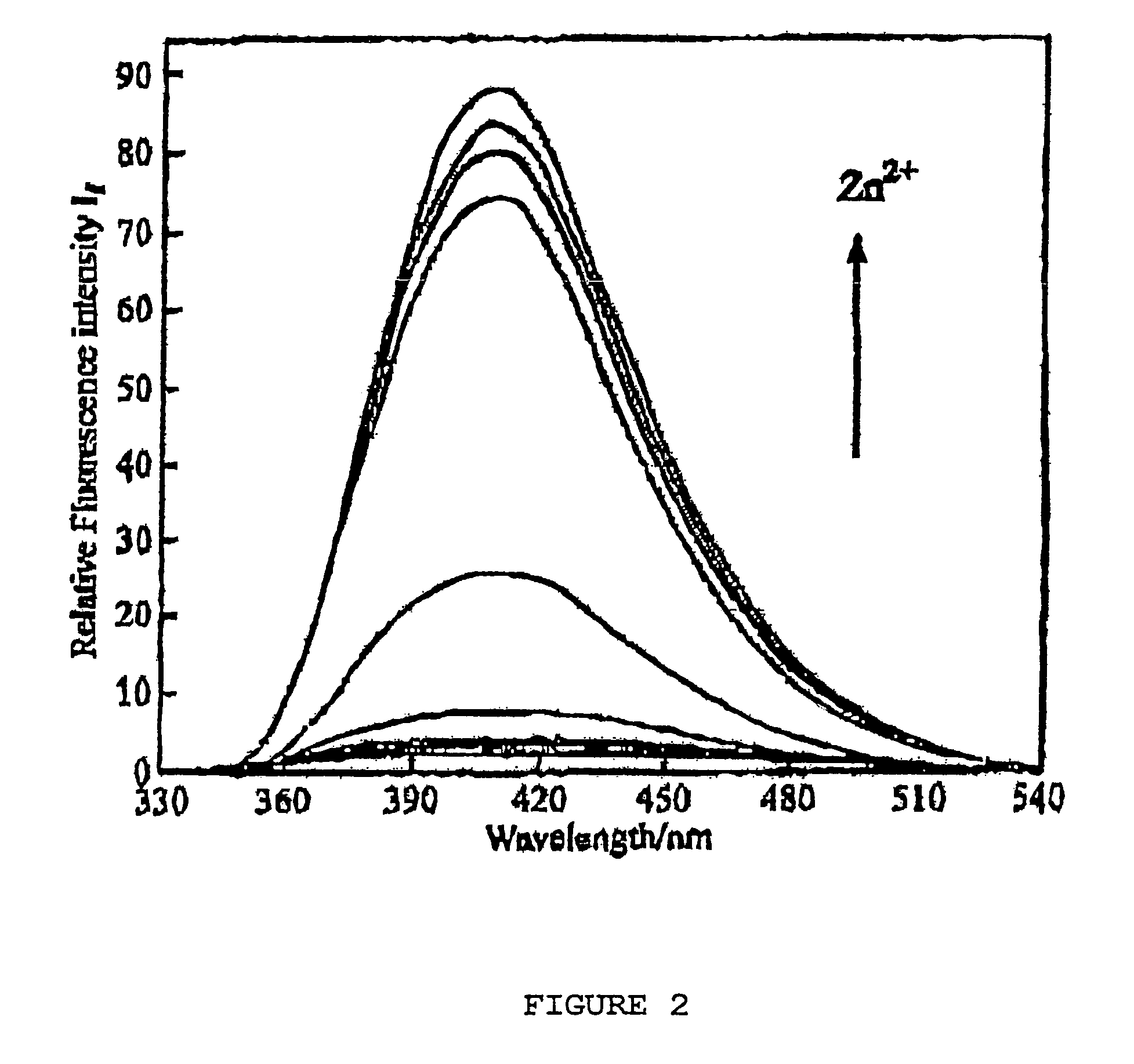
Chiral piperidine and quinucledine ligands
InactiveUS7491544B2High selectivityImprove rigidityAnalysis using chemical indicatorsChemiluminescene/bioluminescenceArylNaphthalene
Zn(II) is selectively detected in a sample by contacting the sample with a tripodal ligand with a piperidine or quinuclidine scaffold, one of which acts as a zinc sensor, in which the rigidity of the ligand scaffold is increased. The rigidity of the ligand scaffold can be increased by adding aromatic groups or cyclic hydrocarbon groups. Examples of aromatic groups include naphthalene and the like. Examples of cyclic groups include nitrogen-substituted cyclohexane and cyclohexene such as piperidine.
Owner:NEW YORK UNIV
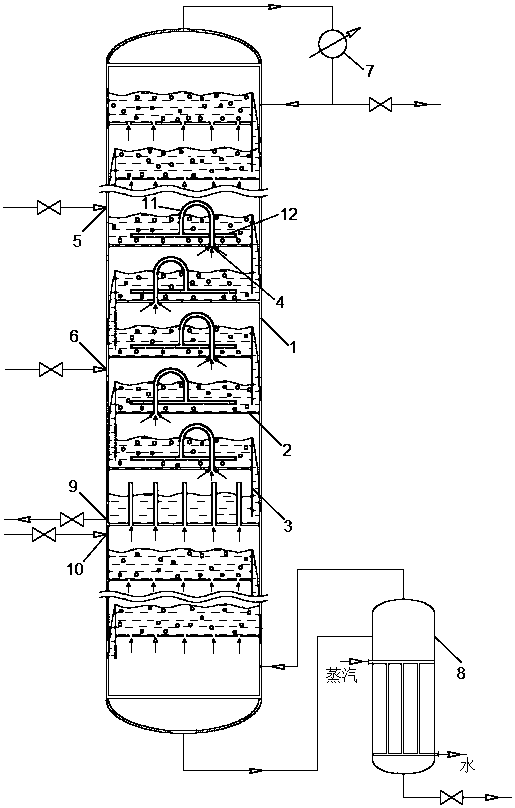
Reactive distillation method for preparing cyclohexanol through hydration of cyclohexene and device of method
InactiveCN109651081AImprove solubilityIncrease conversion rate per passOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsMolecular sieveCyclohexene
The invention relates to a reactive distillation method for preparing cyclohexanol through hydration of cyclohexene and a device of the method. According to the reactive distillation method and the device thereof, a ZSM-5 molecular sieve is adopted as a reaction catalyst, cyclic crown ether or tertiary amine is adopted as a phase transfer catalyst, and a tower plate of a reactive distillation tower is a non-porous tower plate combination body composed of non-porous tower plates, U-shaped vapor rise pipes and annular gas distributors in form. By means of the reactive distillation method and thedevice thereof, the sieve plate hole channel blockage problem caused when vapor at the high gas velocity drives the catalyst to impact the bottom of a sieve plate can be solved, the single-pass conversion rate of cyclohexene is increased, and the reaction energy consumption is reduced.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
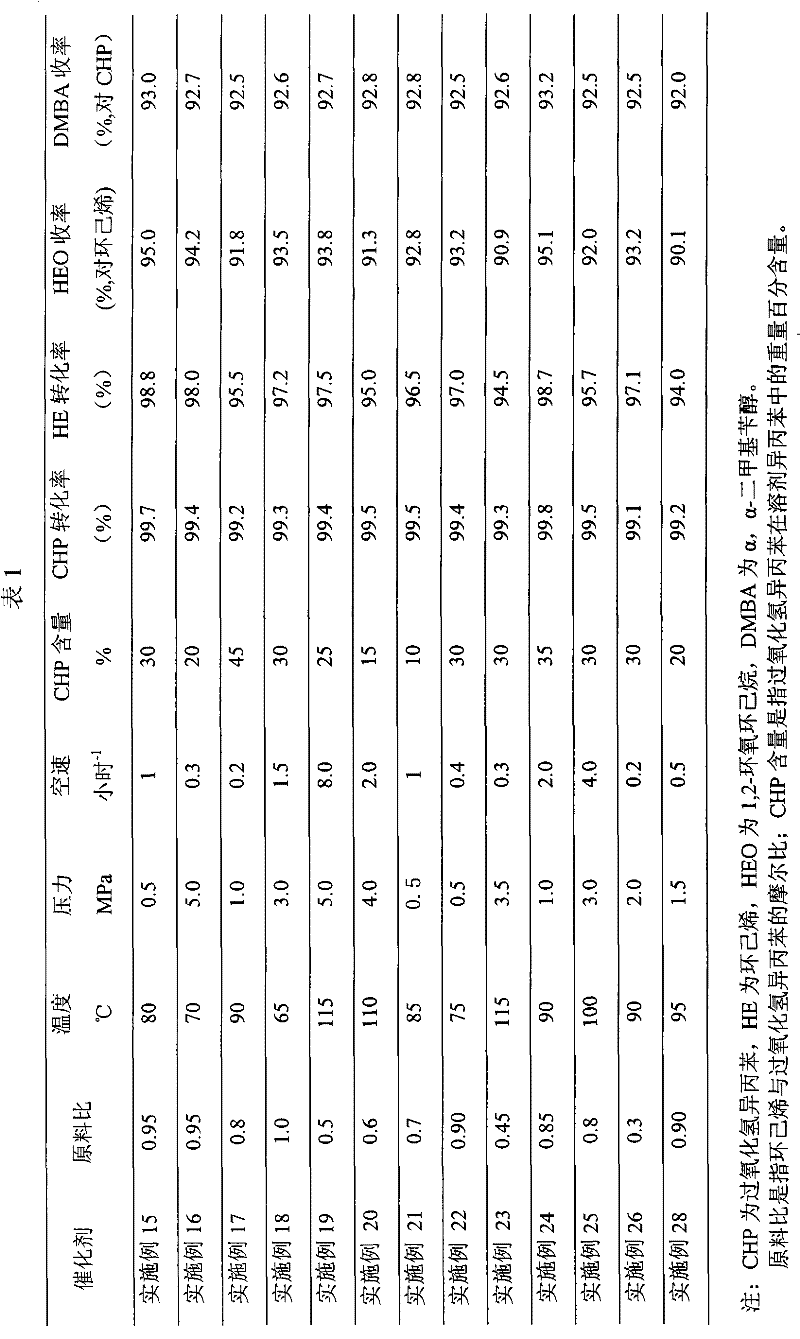
Coproduction method of 1,2-epoxycyclohexane and α,α-dimethylbenzyl alcohol
The invention relates to a method for producing 1,2-epoxy cyclohexane and alpha, alpha-dimethyl benzyl alcohol. The method mainly solves the problems of serious production process pollution, poor product quality and high production cost when the 1,2-epoxy cyclohexane and the alpha, alpha-dimethyl benzyl alcohol are separately produced in the prior art. Isopropyl benzene hydroperoxide and cyclohexene undergo oxidation-reduction reaction on a titanium-containing porous silicon dioxide catalyst under the mild reaction condition, wherein the isopropyl benzene hydroperoxide is reduced into the alpha, alpha-dimethyl benzyl alcohol, and the cyclohexene is oxidized into the 1,2-epoxy cyclohexane; and meanwhile, by adjusting the molar ratio of the cyclohexene to the isopropyl benzene hydroperoxide in the raw materials, the cyclohexene is totally converted or has little residue in the reaction process. According to the technical scheme, the problems are well solved, and the method can be used for industrial production of producing the 1,2-epoxy cyclohexane and the alpha, alpha-dimethyl benzyl alcohol.
Owner:CHINA PETROLEUM & CHEM CORP +1
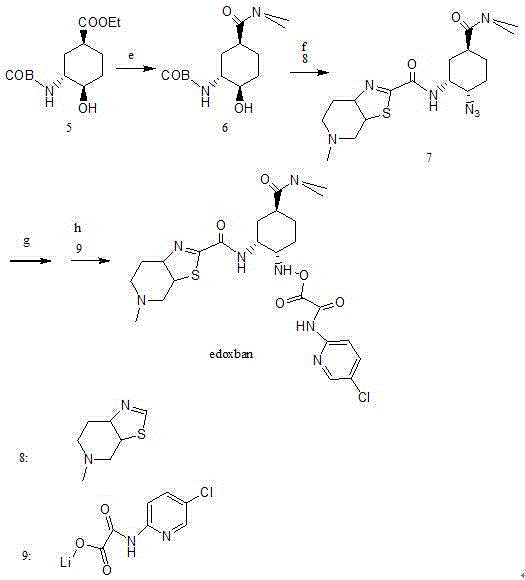
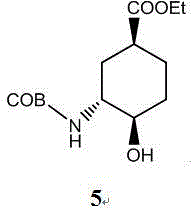
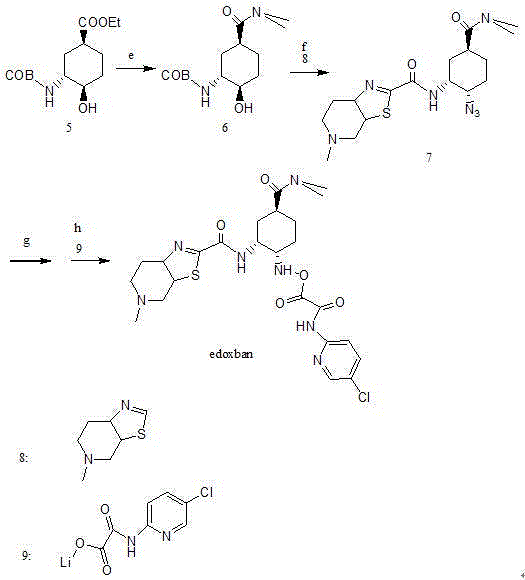
Edoxaban intermediate and preparation method thereof
InactiveCN105198776AStable and reliable quantityHigh yieldCarbamic acid derivatives preparationOrganic compound preparationPtru catalystFormic acid ethyl ester
The invention relates to an edoxaban intermediate and a preparation method thereof. The preparation method of the edoxaban intermediate comprises steps as follows: (a), (1s)-3- cyclohexene-1-formic acid is taken as a raw material, and a compound 2 is generated in the presence of a catalyst, a halogen and a weak base; (b), the compound 2 generates a compound 3 under the action of strong base in an absolute ethanol solution; (c), the compound 3 generates a compound 4 in an ammonia and ethanol solution; (d), the compound 4 reacts with a protecting group of amino under the action of a catalyst, and a compound 5, (1S,3R,4R)-3-[(t-butyloxycarboryl)amino]-4-hydroxycyclohexyl ethyl formate, is generated. Raw materials and reagents required in the synthesis method of the edoxaban intermediate are easy to obtain, the yield is high, the cost is low, reaction conditions are mild, three wastes are relatively fewer, and the quality of the product is stable and reliable.
Owner:天津药物研究院药业有限责任公司
Method for synthesizing 1,2-cyclohexanediol by cyclohexene under selenium catalysis
InactiveCN102503774AImproved Catalytic Oxidative Hydrolysis ReactionReduce dosagePreparation by oxidation reactionsChemical recyclingPtru catalystDistillation
The invention provides a method for synthesizing 1,2-cyclohexanediol by cyclohexene under selenium catalysis. The method comprises the following steps of: taking cyclohexene as a raw material, taking a selenium compound as a catalyst, and taking hydrogen peroxide as an oxidant; carrying out reaction at a temperature of 15-80 DEG C in the presence of a solvent, wherein a mole ratio of the hydrogen peroxide to the cyclohexene is (0.5-1.5): 1, the mole fraction of the selenium compound and the cyclohexene is 0.1-1 percent, and the concentration of the reaction solution based on a solvent calculation is 1-20 mol / L. Furthermore, the production can be continuously carried out by adding the raw materials for the next turn into reaction residues. After the reaction, the solvent and non-reacted raw materials are respectively recycled by distillation and a product is refined. The selenium compound is selected from diselenide, selenious acid, and phenyl, fluoro-phenyl, tolyl substitutes and the like of the diselenide amd the selenious acid. The solvent is selected from acetonitrile, water, ethanol and acetic acid. The method disclosed by the invention has the advantages of simple process flow, temper reaction conditions, and high yield (the highest yield can be up to 92 percent). The method is efficient, clean and environment-friendly and further has the characteristics of simple components in a reaction system, easiness of product purification, and easiness of recycling the solvent and the catalyst.
Owner:JIANGSU YANGNONG CHEM GROUP +1
Method for preparing cyclohexene oxide through hydrogen peroxide epoxidation
InactiveCN101613330AReduce hydrolysisHigh selectivityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsDistillationCyclohexene oxide
The invention relates to a method for preparing cyclohexene oxide by directly taking hydrogen peroxide as an oxygen source and adopting the heteropolyacid salt mixture with reaction-controlled phase transfer catalysis property to catalyze cyclohexene epoxidation. Cyclohexene reacts with hydrogen peroxide under the action of a reaction-controlled phase transfer catalyst; through the regulation of additives, the conversion rate of cyclohexene is greater than 96 percent; the selectivity of hydrogen peroxide to cyclohexene is greater than 98 percent; after reaction ends, the hydrogen peroxide is separated and purified through atmospheric distillation; and the catalyst can be separated and recycled after the reaction ends.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
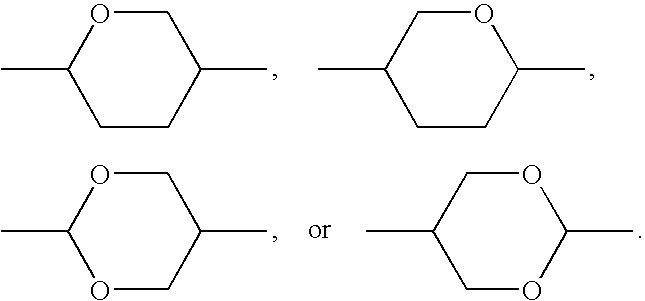


![3-Pyrrolo[b]cyclohexylene-2-dihydroindolinone derivatives and uses thereof 3-Pyrrolo[b]cyclohexylene-2-dihydroindolinone derivatives and uses thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c8d76f7b-66d8-4028-abf5-46afb54c8979/US08084621-20111227-C00001.png)
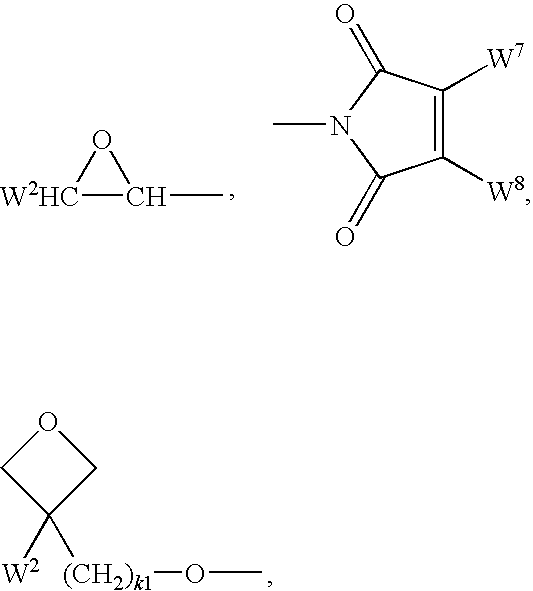
![3-Pyrrolo[b]cyclohexylene-2-dihydroindolinone derivatives and uses thereof 3-Pyrrolo[b]cyclohexylene-2-dihydroindolinone derivatives and uses thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c8d76f7b-66d8-4028-abf5-46afb54c8979/US08084621-20111227-C00002.png)
![3-Pyrrolo[b]cyclohexylene-2-dihydroindolinone derivatives and uses thereof 3-Pyrrolo[b]cyclohexylene-2-dihydroindolinone derivatives and uses thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c8d76f7b-66d8-4028-abf5-46afb54c8979/US08084621-20111227-C00003.png)