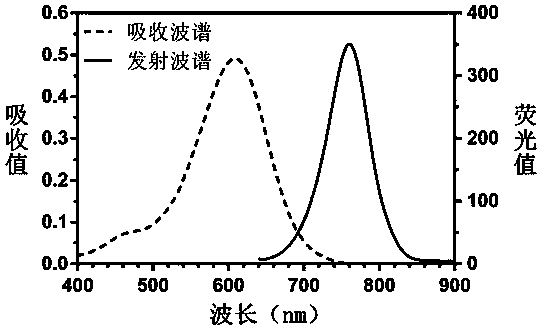
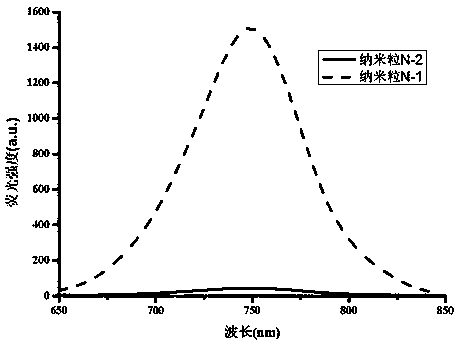
Heptamethine cyanine active fluorescent probe and preparation method and application thereof
A technology of fluorescent probes and cyanines, which is applied in the field of active fluorescent probes of heptamethine cyanines and its preparation and application, can solve the problems of increased synthesis complexity and difficulty of separation and purification, a single type of active site, and inability to meet the requirements of proteins. etc. to simplify the preparation and purification process, reduce complexity, and increase fluorescence intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1 contains cyclohexene chlorine bridging parent dye 1-a synthesis
[0035] According to the synthesis route shown in the figure below, the parent dye 1-a containing cyclohexene chlorine bridge is synthesized, and the specific steps are as follows:
[0036]
[0037] (1) Intermediate N-ethyl-2,3,3-trimethyl-3H-indoline
[0038]Add 2,3,3-trimethyl-3H-indoline (25mL, 24.8g, 156mmol) in a 250mL round bottom flask, disperse in 50mL anhydrous toluene, add iodoethane (30g, 192mmol) dropwise Anhydrous toluene solution was refluxed for 12 h under the protection of argon, and the reaction was terminated after heating was stopped. The solid product was obtained by filtration, washed three times with anhydrous diethyl ether and dried in vacuo to obtain a pink solid with a yield of 79%.
[0039] (2) Synthesis of condensing agent 2-chloro-1-formyl-3-hydroxymethylcyclohexene
[0040] Under ice-bath conditions, add 10 mL of phosphorus oxychloride dropwise to 10 mL of an...
Embodiment 2
[0043] Synthesis and characterization of embodiment 2 target fluorescent probe 2-a-a
[0044]
[0045] Add 200 mg of parent dye 1-a and 1.5 times the molar amount of nucleophile N-(3-aminopropyl) methacrylamide hydrochloride and 3.0 times the molar amount of DIPEA in a 50 mL round bottom flask, and use 20 mL of anhydrous Disperse with methanol, and react in the dark at 50°C for 12 hours. After the reaction solution is cooled to room temperature, it is dropped into 200 mL of anhydrous ether, and a large amount of solid precipitates are precipitated. After filtration, it is vacuum-dried. Using dichloromethane / methanol as the eluent, the 2-a-a dye was obtained by medium-pressure flash preparative chromatography with a yield of 75%.
[0046] fluorescent probe 2-a-a, 1 H NMR (400MHz, DMSO-d6): δ = 8.73 (s, 1H, NH); 8.45 (s, 1H, NH); 8.36 (d, 2H, CH); 7.40 (d, 2H, ArH); 7.37 ( t,ArH,2H); 7.37(t,2H,ArH); 7.16(d,2H,ArH); 6.25(d,2H,CH); 5.72(s,1H,CH); ); 4.24(q,CH 2 ,4H); 3.85(t...
Embodiment 3
[0047] Synthesis and characterization of embodiment 3 target fluorescent probe 2-a-b
[0048]
[0049] The synthesis method is the same as in Example 2, and the nucleophile uses 2-(2-pyridyldithio)ethylamine hydrochloride.
[0050] fluorescent probe 2-a-b, 1 H NMR (400MHz, DMSO-d6): δ=8.73 (s, 1H, NH); 8.36 (d, 2H, CH); 8.25 (s, 1H, CH); 7.00-8.00 (m, 11H, ArH); 6.25(d,2H,CH); 4.24(q,CH 2 ,4H); 3.49(d,2H,CH 2 ); 3.09 (dd,2H,CH 2 ); 2.85(dd,4H,CH 2 ); 2.45(t,1H,CH,); 1.70(s,12H,CH 3 ); 1.56(m,2H,CH 2 ); 1.44(t,CH 3 ,6H). MS(ESI): m / z calculated: 661.34; found: 661.42.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com