Synthesis method of 8-nitro-2-tetrazol-5-yl-4-oxo-4H-1-benzopyran
A technology of tetrazole-based, synthetic method, applied in the field of chemical pharmacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
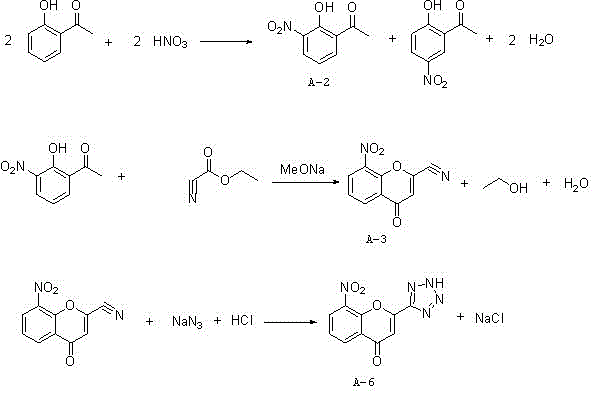
[0028] The synthetic method of 8-nitro-2-tetrazolyl-4-carbonylbenzopyran of the present invention, such as figure 1 As shown, using o-hydroxyacetophenone as a starting material, it is first nitrated with nitric acid to generate a mixture of 2-hydroxyl-3-nitroacetophenone and 2-hydroxyl-5-nitroacetophenone, followed by Inorganic alkali is formed into salt for separation and purification, and then hydrolyzed with acid to obtain 2-hydroxy-3-nitroacetophenone (A-2); 2-hydroxy-3-nitroacetophenone (A-2) and cyano Ethyl formate undergoes a cyclization reaction to generate 8-nitro-2-cyano-4-carbonylbenzopyran (A-3), and finally reacts with sodium azide to generate 8-nitro-2-tetrazolium Base-4-carbonylbenzopyran (A-6); the specific process steps are:
[0029] 1) Add o-hydroxyacetophenone, acetic acid and an organic solvent into the reactor, add 63% concentrated nitric acid dropwise under stirring, and stir the reaction after the dropwise addition. After the reaction is completed, add...
Embodiment 1
[0035] In the four-necked bottle, add o-hydroxyacetophenone: 175g, acetic acid: 183g and dichloromethane: 800mL in sequence, heat up to 40 degrees under stirring, slowly add 63% concentrated nitric acid: 165g, control the temperature at 40 degrees, The dropping time is 4-6 hours. After the dropping, keep warm at 40°C for 4-6 hours. After the reaction, add water: 500g and stir for 30 minutes, extract and separate layers, and concentrate the organic layer to obtain 200g of crude product.
[0036] Add the above crude product: 200g, toluene: 900g to the four-necked bottle, raise the temperature to 60-70 degrees, add sodium carbonate: 165g, react at 70-80 degrees for 2-4 hours, cool down to 30-50 degrees, filter, and bake the filter cake After drying, add it to a four-necked bottle, add methanol: 100g, water: 800g, heat up to 50-60 degrees, slowly add acetic acid: 150g dropwise, stir at 60-70 degrees for 2 hours after dropping, drop to room temperature and filter, filter The cake w...
Embodiment 2
[0040] In the four-necked bottle, add o-hydroxyacetophenone: 175g, acetic acid: 183g and dichloroethane: 800mL in sequence, heat up to 39 degrees under stirring, slowly add 63% concentrated nitric acid: 168g, control the temperature at 39 degrees , the dropping time is 6 hours, the dropping is completed, and the temperature is kept at 40 degrees for 4-6 hours. After the reaction, add water: 500g and stir for 30 minutes, extract and separate layers, and the organic layer is concentrated to obtain 201g of crude product.
[0041] Add the above crude product: 201g, xylene: 900g to the four-necked bottle, heat up to 60 degrees, add potassium carbonate: 195g, react at 75 degrees for 3 hours, cool down to 30-50 degrees, filter, dry the filter cake and add to four In the mouth bottle, add methanol: 100g, water: 800g, heat up to 50-60 degrees, slowly add 30% hydrochloric acid: 140g dropwise, stir at 60-70 degrees for 2 hours after dropping, drop to room temperature and filter, and dry t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com