Synthesis method of ethyl tetrazole-5-carboxylate
A technology of ethyl formate tetrazolium and ethyl formate tetrazolium sodium, which is applied in the field of synthesis of 5-ethyl tetrazolium formate, can solve the problems of long reaction time, unfavorable production, low reaction efficiency, etc. Achieve complete reaction, complete separation of impurities, and increase the effect of reaction rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
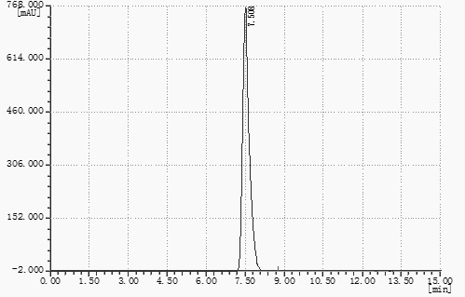
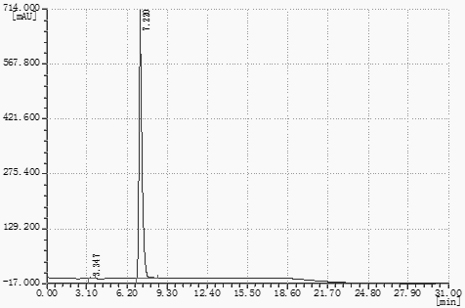
Image
Examples
Embodiment 1
[0035] Add 40g (46.2mL) of toluene, 360g (360mL) of water, and 100g of ethyl cyanoformate into a 1L four-necked flask equipped with a stirring paddle, a condenser, and a thermometer, and start stirring. The mass ratio of ethyl cyanoformate to solvent (mixed solution of toluene and water) is 1:4, slowly add 65g of sodium azide to the reaction system several times, stir and raise the temperature to 90°C, and keep the reaction under reflux for 15h. Monitor the progress of the reaction. After the reaction is detected by TCL, water is added to the system while distilling out the toluene in the system under heat preservation until no toluene stratification remains in the remaining reflux liquid. In the actual operation process, toluene and water will be separated during the distillation process. It is observed that the fraction is not stratified. When the fraction is clarified, there will be no toluene left. As long as the toluene cannot be evaporated, it will be added continuously. ...
Embodiment 2
[0037] Add 40g (46.2mL) of toluene, 360g (360mL) of water, and 100g of ethyl cyanoformate into a 1L four-neck flask equipped with a stirring paddle, a condenser, and a thermometer, and start stirring. Slowly add 72.2 g of sodium azide to the reaction system multiple times, stir and raise the temperature to 90° C., and keep it under reflux for 16 hours. Monitor the reaction process, TCL detects that after the reaction is over, the toluene in the system is distilled out under the heat preservation state, and at the same time, water is replenished in the system in time until there is no toluene layer residue in the remaining reflux liquid. The amount of water added is not strictly limited. Preferably, the amount of water added each time is 30%-40% of the mass of toluene in the remaining reflux liquid. Cool down to 20°C, add 103 g of 36% HCl solution dropwise to the system, neutralize the pH to 3-4, and stir for 30 minutes. Cool down to 15°C, crystallize, and filter with suction....
Embodiment 3
[0039] Add 80g (92.4mL) of toluene, 320g (320mL) of water, and 100g of ethyl cyanoformate into a 1L four-necked flask equipped with a stirring paddle, a condenser, and a thermometer, and start stirring. Slowly add 65 g of sodium azide to the reaction system several times, stir and raise the temperature to 85° C., and keep the reaction under reflux for 14 hours. Monitor the reaction process, TCL detects that after the reaction is over, the toluene in the system is distilled out under the heat preservation state, and water is replenished in the system at the same time, until no toluene stratification remains in the remaining reflux liquid. Cool down to 30°C, add 103 g of 36% HCl solution dropwise to the system, neutralize to pH 3-4, and stir for 30 min. Cool down to 25°C to crystallize, filter with suction, the filter cake is the crude product of ethyl 5-formate tetrazolium, and the filter cake is recrystallized with a mixed solvent of 80% benzene / 20% water (m / m). The mass rati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com