Preparation method of diclazuril impurity A
A technology for diclazuril and impurities, applied in the field of chemistry or medicinal chemistry, can solve problems such as not finding diclazuril impurity A, and achieve the effects of easy availability of starting materials, easy operation and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) Condensation: Add 20 g (66 mmol) of (3-butoxycarbonylamino-3-oxopropionyl)-butyl carbamate, 140 g of dioxane, and 13.6 g of formic acid into a 250 mL four-necked flask, and heat up to 30 ℃, after stirring to dissolve, add the prepared solution of 18.1g sodium nitrite (262mmol) / 36.2g water, and stir and react at 30℃ for 15 hours.
[0028] (2) Decolorization and crystallization: Add 1.0 g of activated carbon to the condensation reaction solution in the previous step, stir for 0.5 hours to decolorize, and then filter with suction. After filtration, the wet product was dried at 50° C. for 16 hours to obtain 18.3 g of off-white diclazuril impurity A dry product with a yield of 83.5%. The content detected by HPLC was 99.6%.
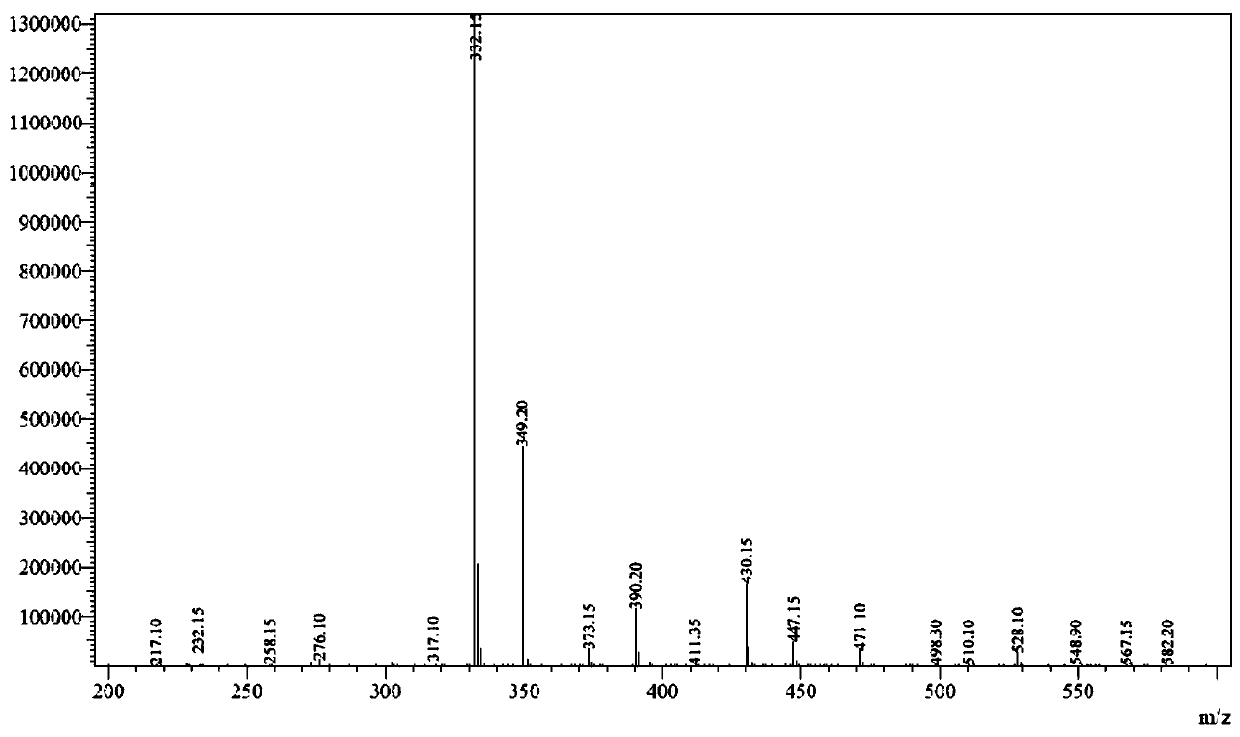
[0029] [M+1]=332.15 (see figure 1 )
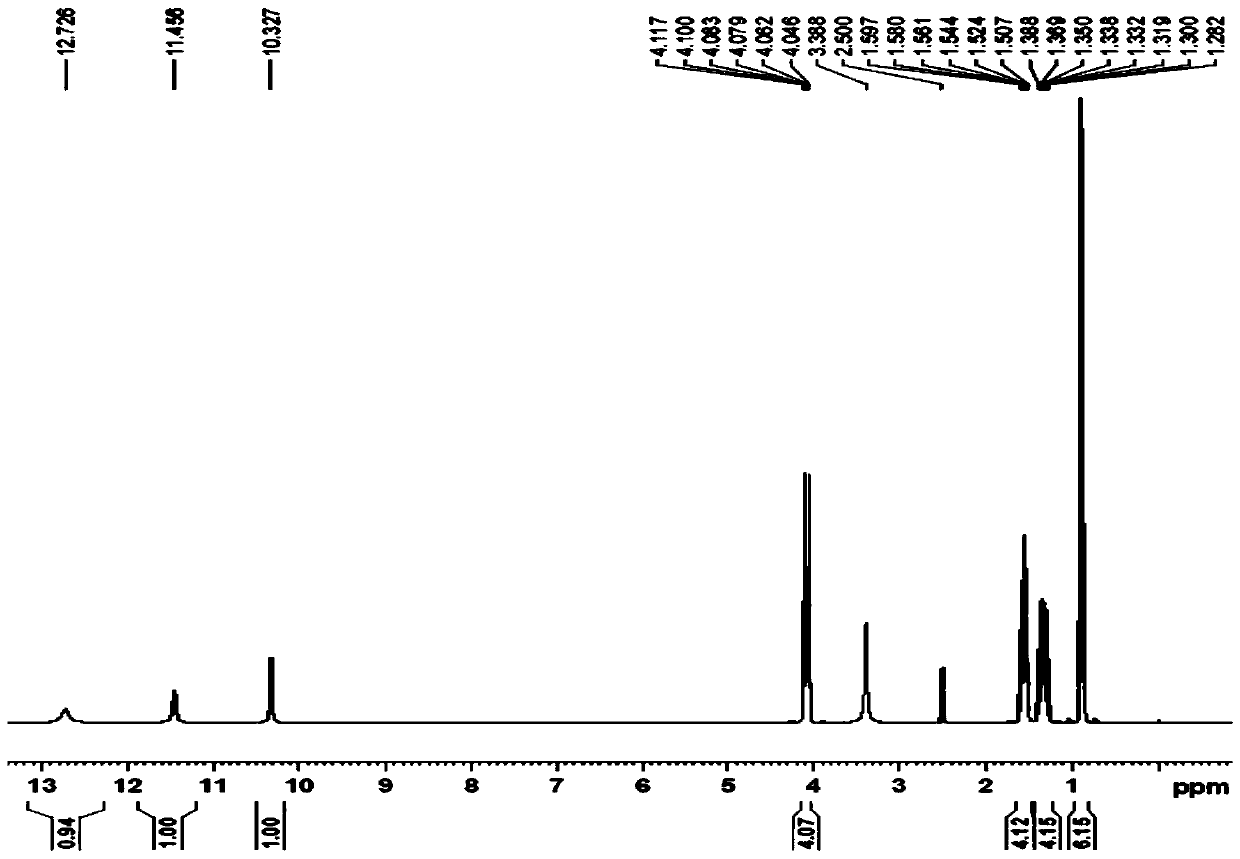
[0030] 1 HNMR(DMSO)δ: 0.96(t,6H),1.28-1.38(m,4H),1.50-1.59(m,4H),4.04-4.11(t,4H),10.32(S,1H),11.45,12.72 (S,2H). (See figure 2 )
Embodiment 2
[0032] (1) Condensation: Add 20 g (66 mmol) of (3-butoxycarbonylamino-3-oxopropionyl)-butyl carbamate, 100 g of dimethyl sulfoxide, and 8.0 g of sulfuric acid into a 250 mL four-necked flask, and heat to 35 ℃, after stirring to dissolve, add the prepared solution of 13.7g sodium nitrite (198mmol) / 36.2g water, and stir and react at 35℃ for 20 hours;
[0033] (2) Decolorization and crystallization: add 1.5 g of activated carbon to the condensation reaction solution in the previous step, stir for 1 hour to decolorize, filter with suction, transfer the filtrate to a 500 mL four-neck flask, add 250 g of methanol dropwise at 35°C, and add dropwise for 1 hour. After suction filtration, the wet product was dried at 80° C. for 12 hours to obtain 18.8 g of off-white diclazuril impurity A dry product with a yield of 85.8%. The content detected by HPLC was 99.7%.
Embodiment 3
[0035] (1) Condensation: Add 20 g (66 mmol) of (3-butoxycarbonylamino-3-oxopropionyl)-butyl carbamate, 100 g of dimethyl sulfoxide, and 4.0 g of acetic acid into a 250 mL four-necked flask, and heat to 40°C, stir to dissolve, then add the prepared 17.5g sodium nitrite (254mmol) / 36.2g water solution, stir and react at 40°C for 10 hours;
[0036] (2) Decolorization and crystallization: Add 1.5 g of activated carbon to the condensation reaction solution in the previous step, stir for 1 hour for decolorization, and filter with suction. After filtration, the wet product was dried at 60° C. for 14 hours to obtain 18.5 g of off-white diclazuril impurity A dry product with a yield of 84.4%. The content detected by HPLC was 99.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com