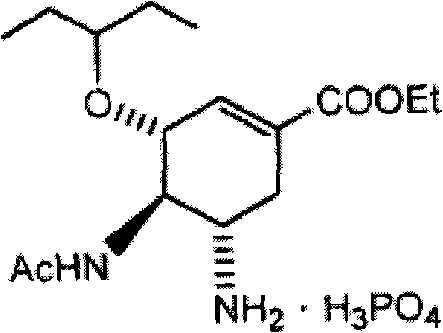
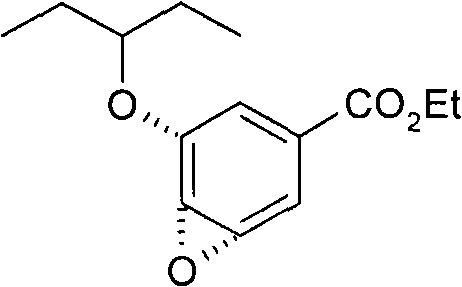
Process for the preparation of (3r,4r,5s)-4,5-epoxy-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylic acid ethyl ester
A technology of ethyl propoxy and ethyl formate, applied in the -4 field, can solve the problems of ring opening of epoxy products, benzene ring impurities, etc., and achieve the effect of reducing the cost of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
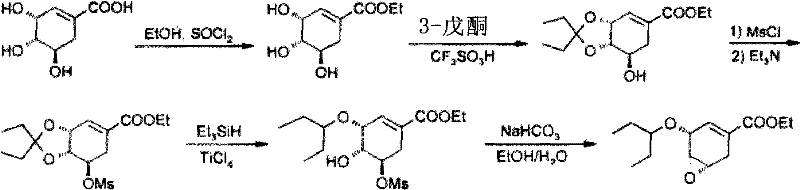
Examples
Embodiment 1
[0027] Preparation of (3R,4S,5R)-3,4,5-trihydroxy-1-cyclohexene-1-carboxylic acid ethyl ester
[0028] At room temperature, shikimic acid (HPLC purity: 95%) (100.0 g, 0.55 mol) was suspended in 400.0 ml of absolute ethanol, and thionyl chloride (19.5 ml, 0.27 mol) was added dropwise with stirring. After the dropwise addition, the mixture was heated to 78°C and refluxed for 3 hours. After the reaction was completed, it was cooled to 40°C and concentrated under reduced pressure to obtain a brown oil.
Embodiment 2
[0030] Preparation of (3R,4S,5R)-3,4-O-isopentylidene-5-hydroxy-1-cyclohexene-1-carboxylic acid ethyl ester
[0031] Under nitrogen protection, triethyl orthoformate (96.5ml, 0.57mol), 3-pentanone (61.5ml, 0.57mol), absolute ethanol (34.0ml, 0.57mol), benzenesulfonic acid (0.2g, 1.26mmol), stirred for 1 hour, and naturally heated to 45°C. In another reaction bottle, add 100ml of absolute ethanol to the brown oil obtained in Example 1, and stir well. The solution obtained above was added dropwise to the ethanol solution of ethyl (3R,4S,5R)-3,4,5-trihydroxy-1-cyclohexene-1-carboxylate, and the stirring was continued for 2 hours. After completion of the reaction, it was concentrated to dryness under reduced pressure to obtain a dark brown oil. Add 500ml of isopropyl acetate to dissolve the oily matter and proceed to the next reaction.
Embodiment 3
[0033] Preparation of (3R, 4S, 5R)-3,4-O-isopentylidene-5-O-(methylsulfonic acid)-1-cyclohexene-1-carboxylic acid ethyl ester
[0034] Cool the isopropyl acetate solution of (3R, 4S, 5R)-3,4-O-isopentylidene-5-hydroxyl-1-cyclohexene-1-carboxylic acid ethyl ester obtained in Example 2 to 0°C , add methanesulfonyl chloride (56.8ml, 0.73mol), and then add triethylamine (160.0ml, 1.15mol) dropwise, and control the temperature not to exceed 10°C. After the dropwise addition, the temperature was naturally raised to room temperature, and stirring was continued for 30 minutes. Filter and wash the filter cake with 100ml of isopropyl acetate. The combined filtrates were washed with 300 ml of water. The separated isopropyl acetate layer was concentrated to dryness under reduced pressure to obtain a yellow oil.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com