Combined medicine capable of simultaneously amplifying immunogenic cell death and enhancing anti-tumor effect
A technology of cell death and immunogenicity, applied in the field of medicine, can solve the problems of immunogenic cell death, and achieve the effect of enhancing phagocytosis and maturation, improving immunogenicity and amplifying cell death
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 Synthesis of 3-(aminopropyl) methacrylamide (amino) modified HPMA polymer
[0043] Mix 30 ml of freshly steamed methacryloyl chloride with 15 ml of dichloromethane, and slowly drop it into 77 ml of dichloromethane solution containing 21 ml of 1-amino-2-propanol and 32 g of sodium carbonate. Warmed to room temperature, stirred for 1h. Then the reaction solution was placed in a low-temperature cold bath at -50°C for 1 h, and a white precipitate was generated. After filtration, the precipitate was recrystallized with acetone, and the white crystal was HPMA monomer.
[0044] With 2, 2'-azobisisobutyronitrile (AIBN) as the initiator and methanol as the solvent, polymer monomers HPMA, 3-(aminopropyl) methacrylamide salt (APMA) (HPMA:APMA= 80: 20 mol%) to generate HPMA polymer precursors by radical polymerization solution reaction (polymer monomer: initiator: solvent = 12.5:2:85.5 wt%).
[0045] AIBN, HPMA and APMA were weighed into ampoules according to the above...
Embodiment 2
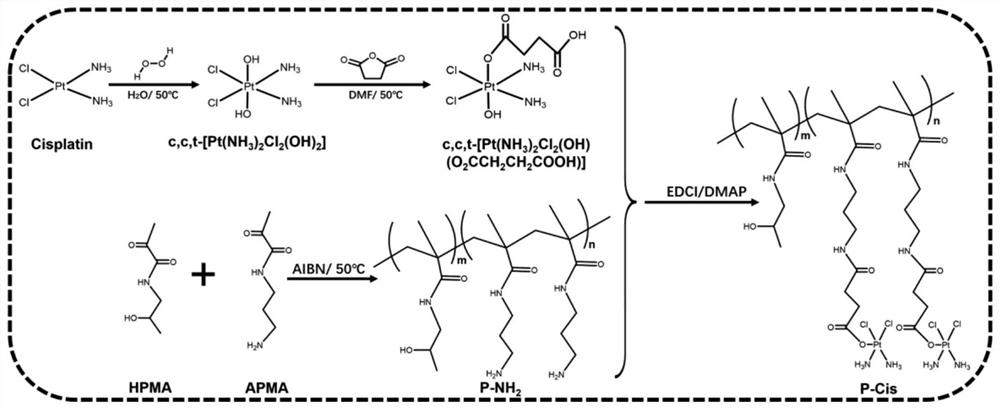
[0046] Example 2 Synthesis of Cytotoxic HPMA Polymer Cisplatin Conjugates
[0047] Disperse 100 mg of cisplatin in 5 ml of deionized water and heat it in a water bath to 50°C, add 3 ml of 30% hydrogen peroxide solution dropwise under stirring and react for 2 hours. Cooled and filtered to obtain light yellow hydroxyl derivatized cisplatin solid c,c,t-[PtCl 2 (OH) 2 (NH 3 ) 2 ].
[0048] Mix 1 ml of dimethyl sulfoxide solution containing 50 mg hydroxyl derivatized cisplatin and dimethyl sulfoxide solution containing 15 mg succinic anhydride, react at 40°C for 24 hours, the reaction solution is precipitated in ether and washed with methanol , the resulting yellow solid was filtered and dried in vacuo to give the carboxyl-derivatized cisplatin solid c,c,t-[PtCl 2 (OH)(NH 3 ) 2 (O 2 CCH 2 CH 2 CO 2 H)].
[0049] Dissolve 20 mg carboxy-derivatized cisplatin, 14.45 mg 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride and 5.56 mg N-hydroxysuccinimide in 200 μl DM...
Embodiment 3
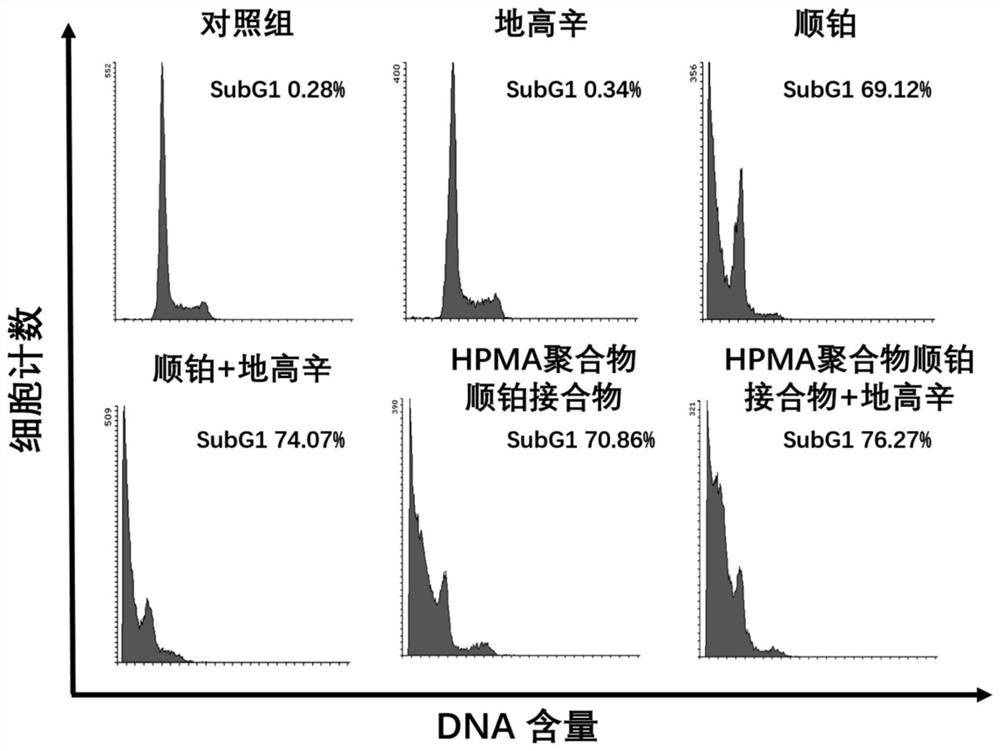
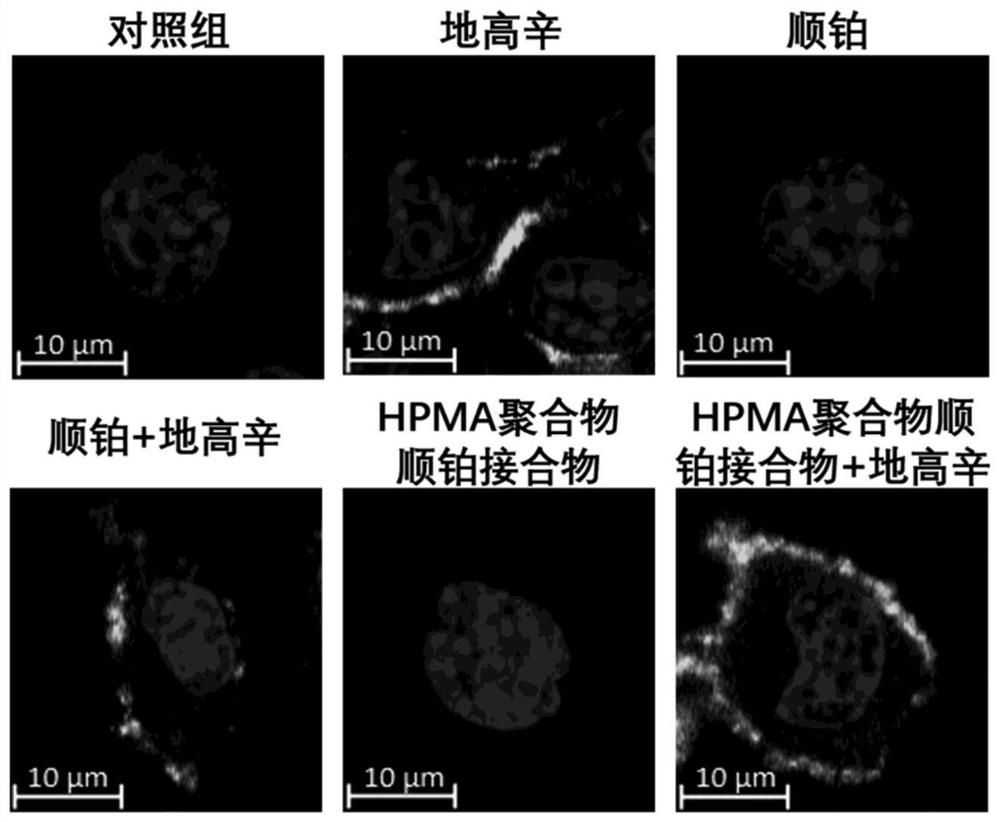
[0050] Example 3 Flow cytometry detection of the effect of HPMA polymer cisplatin conjugate and digoxin on the cell cycle and apoptosis of B16F10 cells
[0051] Melanoma B16F10 cells were seeded into 12-well plates at 5000 / well, and placed in a cell culture incubator to adhere to the wall for 24 hours. Divided into control group, digoxin group, cisplatin group, cisplatin + digoxin group, HPMA polymer cisplatin conjugate group and HPMA polymer cisplatin conjugate group + digoxin group for the test:
[0052] Control group: add 2 ml RPMI1640 medium (containing 10% FBS);
[0053] Digoxin group: Add 2 ml of RPMI1640 medium containing 62.4 μg / ml digoxin (containing 10% FBS);
[0054] Cisplatin group: Add 2 ml of RPMI1640 medium (containing 10% FBS) containing 100 μg / ml cisplatin;
[0055] Cisplatin + digoxin: Add 2 ml of RPMI1640 medium (containing 10% FBS) containing 100 μg / ml cisplatin and 62.4 μg / ml digoxin;
[0056] HPMA polymer cisplatin conjugate group: Add 2 ml of RPMI1640...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com