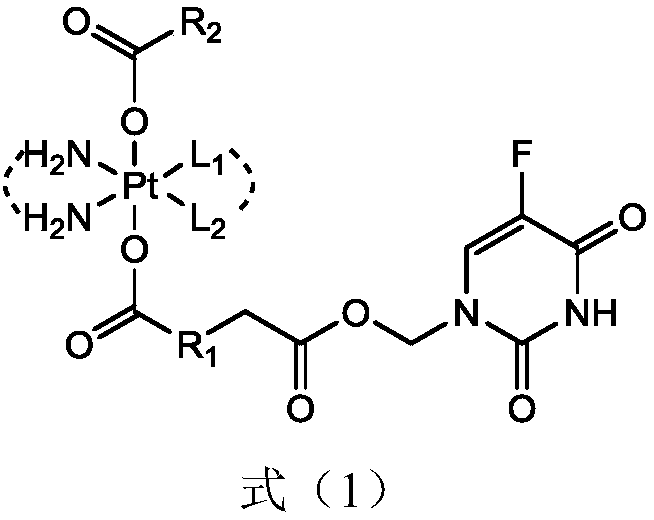
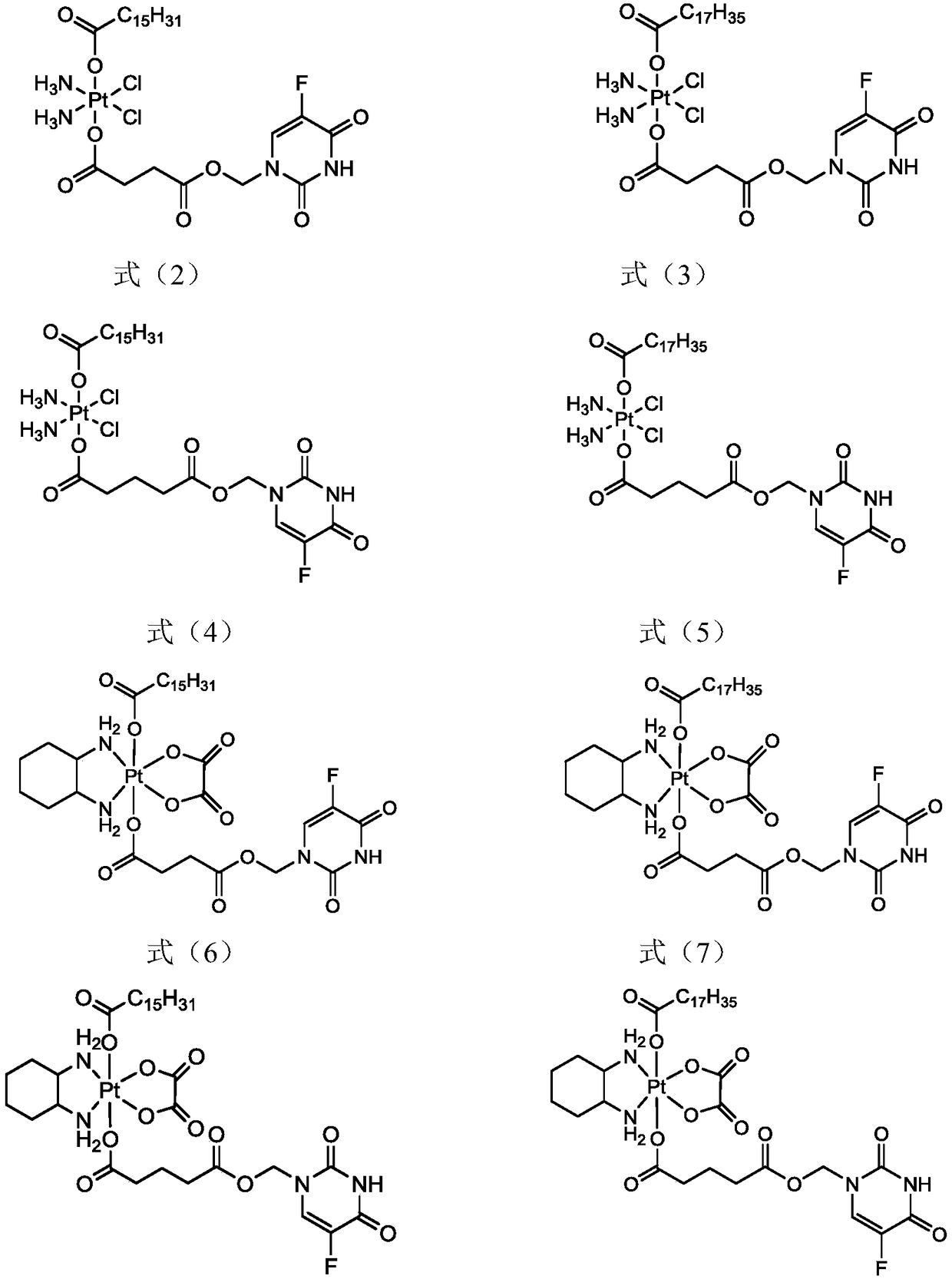
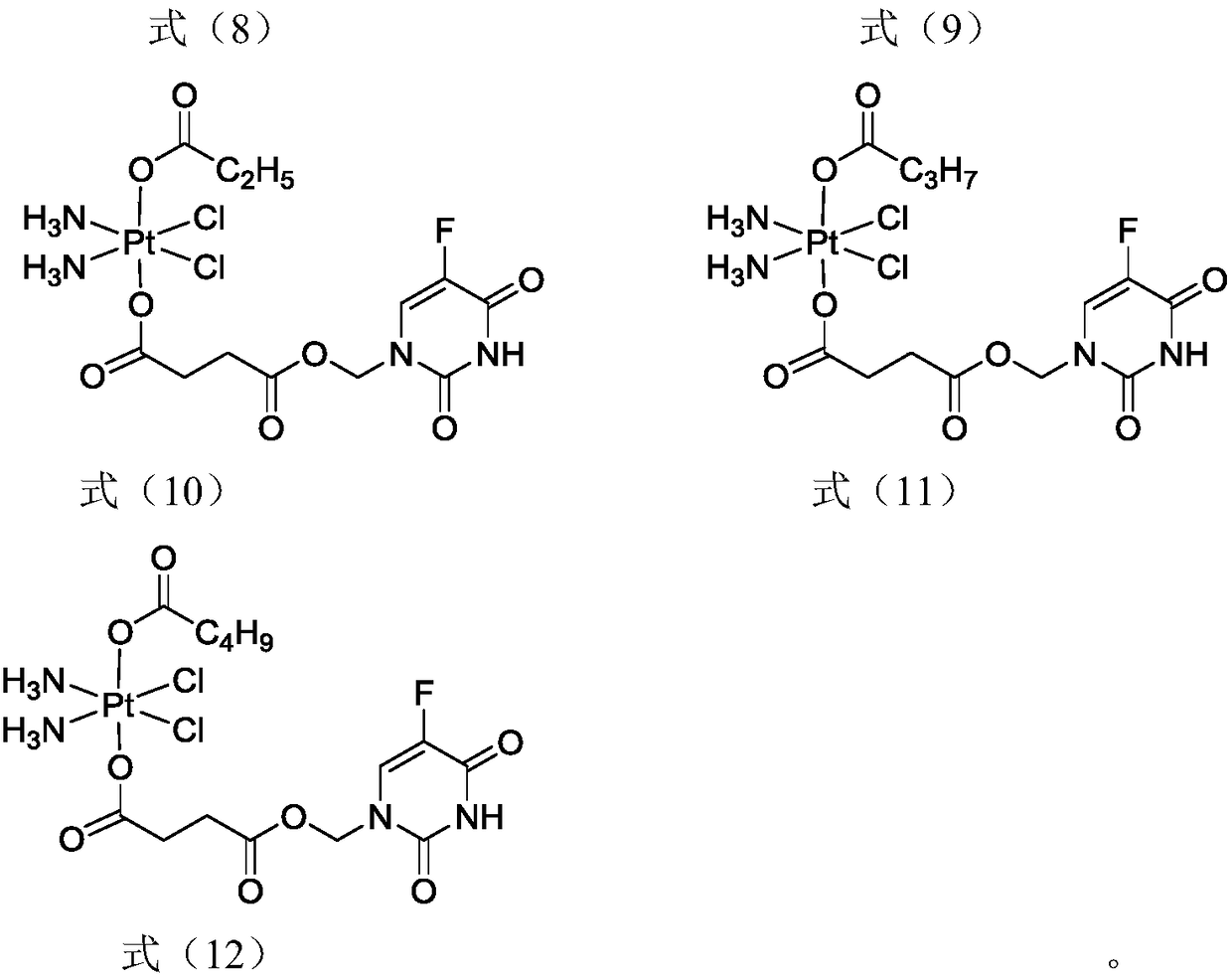
5-fluorouracil-platinum (IV) complex, intermediate, as well as preparation method and application thereof
A technology of fluorouracil and complexes, applied in the field of anticancer chemical drugs, can solve the problems of restricted application and development of ototoxicity and neurotoxicity, and achieve the effects of obvious cell selectivity, simple operation and good anti-proliferation ability of the compound.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0084] The preparation method of formula (14) compound comprises:
[0085] 5-fluorouracil is reacted with formaldehyde to obtain a compound of formula (17);
[0086] The compound of formula (17) and the compound of formula (18) undergo an esterification reaction in the presence of the second condensing agent and the second acid-binding agent to obtain the compound of formula (14)
[0087]
[0088] Wherein, the second condensing agent is DMAP, and HOBT and EDCI can also be used;
[0089] The second acid binding agent is triethylamine, or adopts pyridine.
[0090] The above-mentioned 5-fluorouracil-platinum (IV) complex can be applied in the preparation of anti-tumor, breast cancer, colon cancer, cervical cancer or lung cancer drugs, and has a synergistic sensitization effect, and the IC50 value is significantly lower than that of cisplatin, oxali Platinum, 5-FU and combined administration have shown to have good anti-proliferation ability on cancer cells, and the lethality...
Embodiment 1
[0093] The structural formula of the 5-fluorouracil-platinum (IV) complex a of the present embodiment is as follows:
[0094]
[0095] The synthesis route of the preparation method of the 5-fluorouracil-platinum (IV) complex described in this example is as follows:
[0096]
[0097]
[0098] 1. Preparation of compound a2
[0099] Accurately weigh 5-fluorouracil (2.20g, 17.0mmol) into a 50mL round-bottomed flask, add 37% formaldehyde solution (2.06g, 37.8mmol), heat and reflux at 60°C for 2 hours, and obtain a transparent solution by rotary evaporation after 2 hours. Viscose a1 (78% yield). Add 20mL of anhydrous acetonitrile to compound a1, add succinic anhydride (2.18g, 21.8mmol), 4-dimethylaminopyridine DMAP (0.12g, 0.96mmol) and triethylamine (1.42mL, 10mmol) successively under stirring , react overnight at 50°C. After the reaction was detected by TCL, the solvent was removed by rotation, purified by a silica gel column, crystallized from absolute ethanol to obta...
Embodiment 2
[0124] The structural formula of the 5-fluorouracil-platinum (IV) complex b of the present embodiment is as follows:
[0125]
[0126] The synthesis route of the preparation method of the 5-fluorouracil-platinum (IV) complex described in this example is as follows:
[0127]
[0128] 1. Preparation of compound b
[0129] Accurately weigh compound a4 (115.0mg, 0.20mmol) and place it in a 10mL round-bottomed flask, add 2mL of ultra-dry DMF to it, add stearic anhydride (165.4mg, 0.30mmol) under magnetic stirring, and protect from light with argon for 60 °C overnight. TCL detection After the reaction was completed, the DMF was removed by rotary evaporation in a water bath at 60°C, the residue was dissolved in 1 mL of methanol, and a silica gel plate was prepared for purification to obtain a light yellow solid. After washing with anhydrous ether to remove residual DMF, the product was vacuum-dried to obtain 50.0 mg of compound b with a yield of 29.7%.
[0130] pass 1 H NMR...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com