Nedaplatin medical composition and application
A nedaplatin and composition technology, applied in the field of nedaplatin pharmaceutical composition, can solve the problems of high water content, poor clarity, difficult freeze-drying of nedaplatin, etc., to achieve improved curative effect, good clarity, and not easy to decompose Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
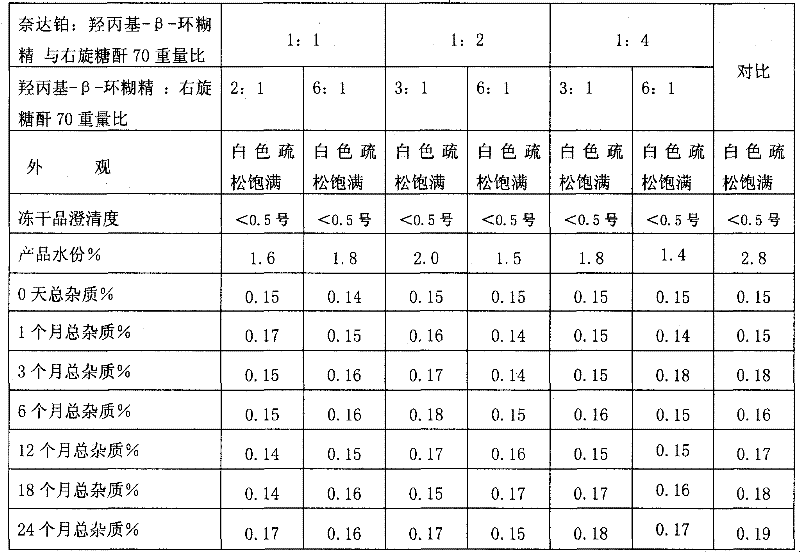
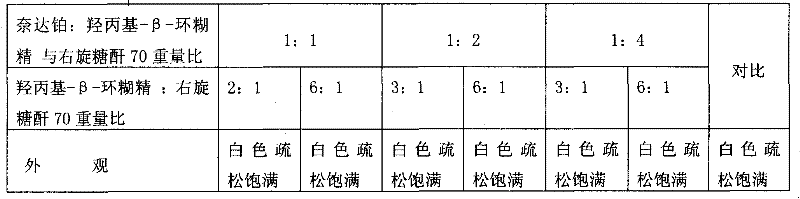
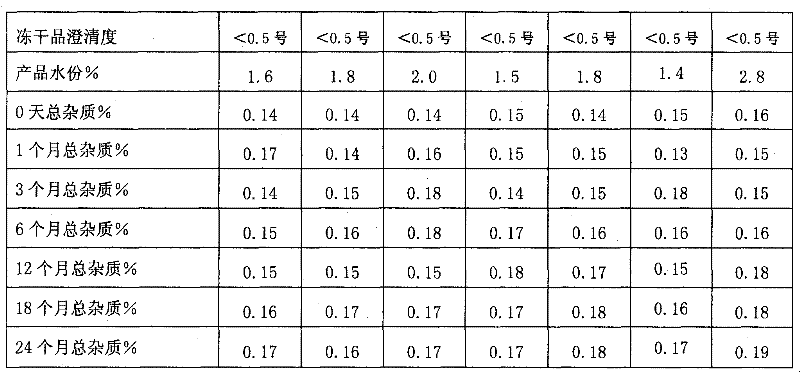
[0049] Weigh 25g of hydroxypropyl-β-cyclodextrin, add 70 5g of dextran to 1800ml of sterile water for injection and stir to dissolve, add activated carbon to absorb to remove heat source, add nedaplatin to stir and dissolve, add to 2000ml, equipped with 0.22 micropore Membrane filtration, filling in sterile vials. Freeze in a freeze-drying cabinet at -45°C to -55°C for 2 hours, sublimate and dry at -10°C to 0°C for 12 hours, and then dry at 35°C for 10 hours, fill the box with nitrogen, seal it with a plug, and pack it.
Embodiment 2
[0051] Weigh 25g of hydroxypropyl-β-cyclodextrin, add 705g of dextran to 800ml of sterile water for injection and stir to dissolve, add activated carbon to absorb to remove the heat source, add nedaplatin to stir and dissolve, add to 1000ml, equipped with 0.22 micropore Membrane filtration, filling in sterile vials. Freeze in a freeze-drying cabinet at -45°C to -55°C for 2.5 hours, sublimate and dry at -10°C to 0°C for 15 hours, dry at 35°C for 8 hours, press the plug, take it out of the box, seal it with a cap, and pack it.
Embodiment 3
[0053] Weigh 25g of hydroxypropyl-β-cyclodextrin, add 705g of dextran to 800ml of sterile water for injection and stir to dissolve, add activated carbon to absorb to remove the heat source, add nedaplatin to stir and dissolve, add to 1000ml, equipped with 0.22 micropore Membrane filtration, filling in sterile vials. Place the sterile solution in a large plate and put it in a freeze-drying cabinet to freeze at -45°C to -55°C for 3 hours, sublimate and dry at -10°C to 0°C for 20 hours, and then dry at 35°C for 6 hours, then fill it with nitrogen gas out of the box, and pulverize . Determine the content of nedaplatin in the freeze-dried powder, divide and pack into vials according to the converted weight of nedaplatin, add stoppers, press caps and seal, and pack.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com