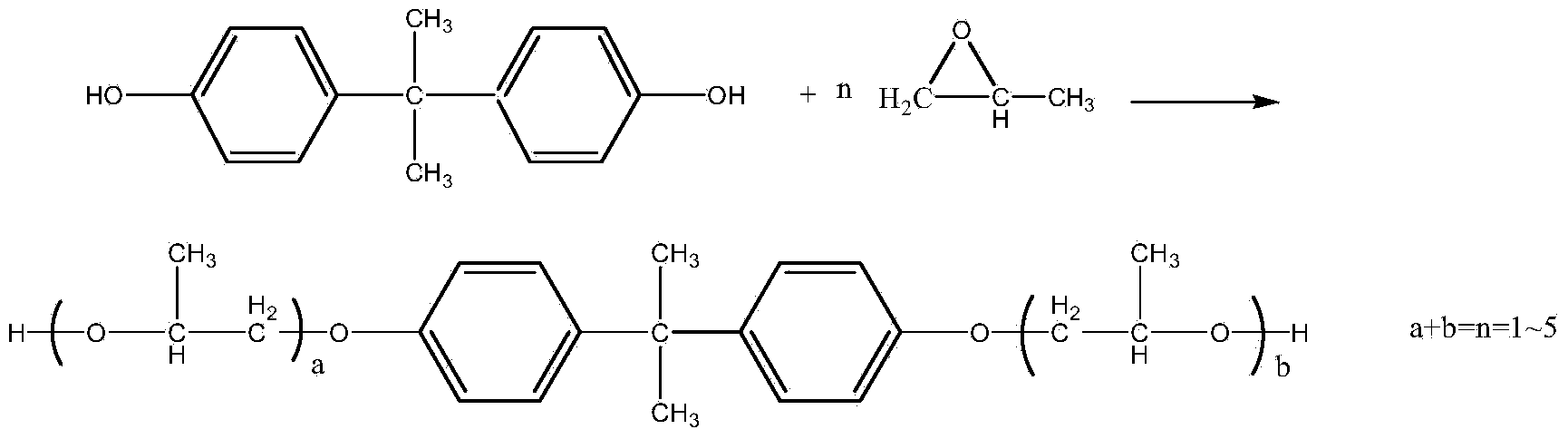
Method for synthesizing dihydroxypropyl bisphenol A ether through one-step process
A technology of bishydroxypropyl bisphenol and step method, applied in the field of organic compound synthesis, can solve the problems of poor quality, high content of by-products, isomerization of propylene oxide and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Add 684g of bisphenol A and 1.0g of 1,1'-bis(diphenylphosphino)ferrocene into the reaction kettle, vacuumize with a vacuum pump, and use N 2 Replace the air in the reactor. After three replacements, the vacuum degree ≥ -0.096MPa, N 2 Under protection, turn off the vacuum and heat up the material; when the bisphenol A is completely melted, vacuumize at this temperature for 20 minutes, after removing the low boiling point substances, continue to add 348g of propylene oxide, and control the reaction temperature at 160-165°C , The pressure inside the reactor is -0.04~0.3Mpa. After the addition, keep warm and continue the reaction until the pressure no longer drops; after the reaction is completed, cool down to 100°C and use vacuum degassing for 20 minutes, then discharge to obtain the finished product.
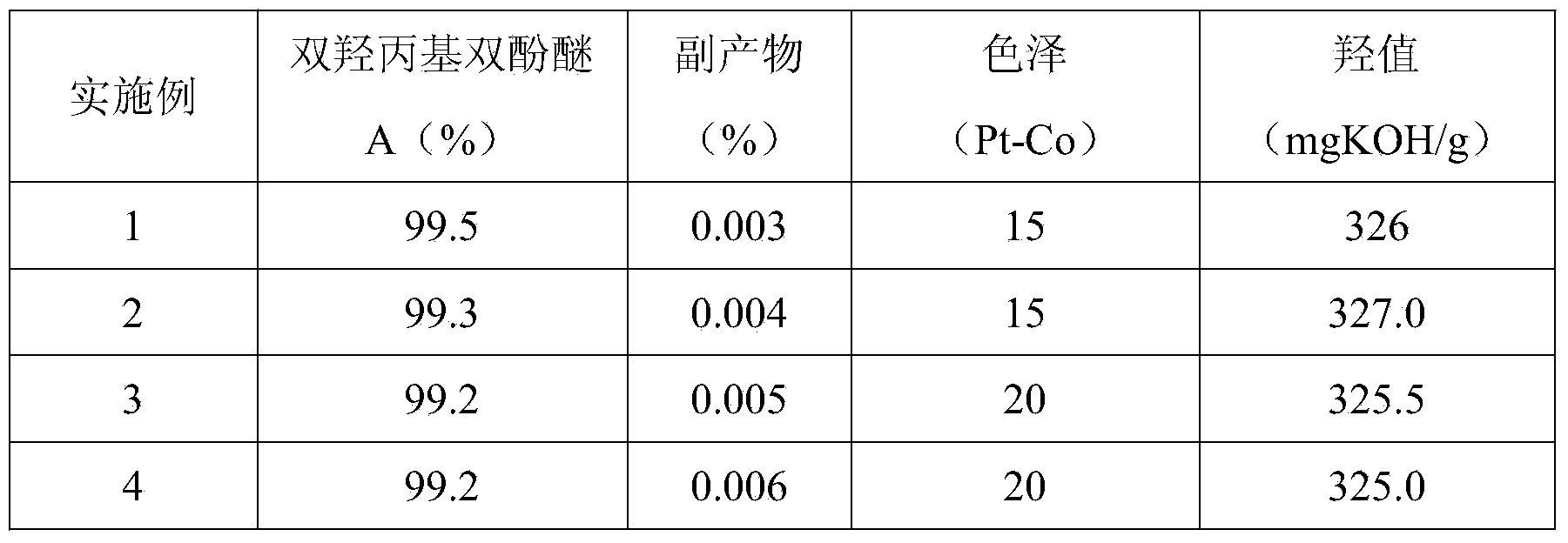
[0027] The product was analyzed by liquid chromatography: the content of propylene alcohol by-products was 0.003%, and the bishydroxypropyl bisphenol A ether was 99.5%. The...
Embodiment 2
[0029] Add 684g of bisphenol A and 0.5g of 1,1'-bis(diphenylphosphino)ferrocene into the reaction kettle, vacuumize with a vacuum pump, and use N 2 Replace the air in the reactor. After three replacements, the vacuum degree ≥ -0.096MPa, N 2 Under protection, turn off the vacuum, and raise the temperature to carry out the compounding; when the bisphenol A is completely melted, vacuumize at this temperature for 20 minutes, after removing the low boiling point substances, continue to add 355g of propylene oxide, and control the reaction temperature at 165-170°C , The pressure inside the reactor is -0.04~0.3Mpa. After the addition, keep warm and continue the reaction until the pressure no longer drops; after the reaction is completed, cool down to 100°C and use vacuum degassing for 20 minutes, then discharge to obtain the finished product.
[0030]The product was analyzed by liquid chromatography: the by-product content of propylene alcohol was 0.004%, and the bishydroxypropyl bis...
Embodiment 3
[0032] Add 684g of bisphenol A and 0.3g of 1,1'-bis(diphenylphosphino)ferrocene into the reaction kettle, vacuumize with a vacuum pump, use N 2 Replace the air in the reactor. After three replacements, the vacuum degree ≥ -0.096MPa, N 2 Under protection, turn off the vacuum, and heat up the material; when the bisphenol A is completely melted, vacuumize at this temperature for 20 minutes, after removing the low boiling point substances, continue to add 420g of propylene oxide, and control the reaction temperature at 170-175°C , The pressure inside the reactor is -0.04~0.3Mpa. After the addition, keep warm and continue the reaction until the pressure no longer drops; after the reaction is completed, cool down to 100°C and use vacuum degassing for 20 minutes, then discharge to obtain the finished product.
[0033] The product was analyzed by liquid chromatography: the content of propylene alcohol by-products was 0.005%, and the bishydroxypropyl bisphenol A ether was 99.2%; the co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hydroxyl value | aaaaa | aaaaa |
| Hydroxyl value | aaaaa | aaaaa |
| Hydroxyl value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com