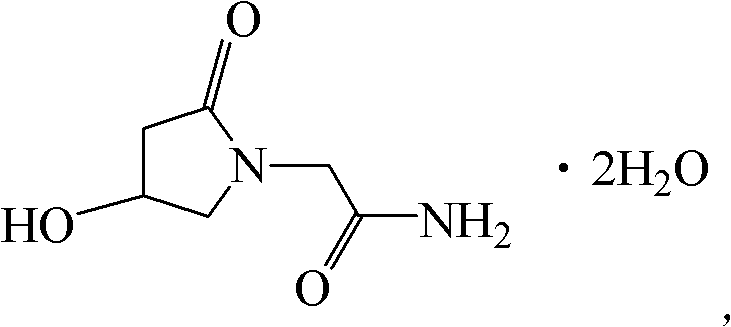
Oxiracetam compound and pharmaceutical composition thereof
A compound and composition technology, applied in the field of medicine, can solve the problems of reduced solubility of preparations, unstable structure, poor resolubility of solid dosage forms, etc., and achieve the effect of less content and controllable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Add 50g of oxiracetam, 600ml of N,N-dimethylformamide and 300ml of ethylene glycol into the liquid mixing tank, stir until they are completely dissolved, adjust the pH value of the liquid to 4.5 with triethylamine or acetic acid, and then add Activated carbon, stirred and adsorbed, decarburized by filtration with 0.45μm microporous membrane, and then sterilized with 0.2μm membrane for the first time and terminal sterilization filtration with 0.2μm membrane for the second time to obtain a clarified solution, and then the clarified solution was transferred to the pressure In the container, seal the pressure vessel, heat it in a water bath at 60-70°C, and adjust the pressure inside the pressure vessel to 1Mpa. Under this condition, slowly add 1800ml of isopropyl ether to produce a white precipitate, take out the mixture in the pressure vessel, and filter , washed with methanol and deionized water in sequence, and dried under reduced pressure for 3 hours to obtain the oxirac...
Embodiment 2
[0042] Add 50g of oxiracetam, 675ml of N,N-dimethylformamide and 225ml of ethylene glycol into the liquid mixing tank, stir until they are completely dissolved, adjust the pH value of the liquid to 5.0 with triethylamine or acetic acid, and then add Activated carbon, stirred and adsorbed, decarburized by filtration with 0.45μm microporous membrane, and then sterilized with 0.2μm membrane for the first time and terminal sterilization filtration with 0.2μm membrane for the second time to obtain a clarified solution, and then the clarified solution was transferred to the pressure In the container, seal the pressure vessel, heat it in a water bath at 60-70°C, and adjust the pressure in the pressure vessel to 1Mpa. Under this condition, slowly add 2700ml of isopropyl ether to produce a white precipitate, take out the mixture in the pressure vessel, and filter , washed with methanol and deionized water in sequence, and dried under reduced pressure for 3 hours to obtain the oxiracetam...
Embodiment 3
[0047] Using the oxiracetam compound prepared in Example 1 or 2 of the present invention and commercially available mannitol as raw materials, the prescription of the pharmaceutical composition in Table 1 is adopted:
[0048] Table 1
[0049]
[0050] A total of 1000 bottles were made
[0051] Preparation Process:
[0052] Under aseptic conditions, accurately weigh the oxiracetam compound and sterile mannitol according to the prescriptions of the first group to the fourth group in Table 1, and place the oxiracetam compound and sterile mannitol in a solid powder mixer After uniform mixing, the obtained raw materials are transferred to the aseptic preparation workshop, accurately measured and distributed into 1000 bottles, and capped to obtain the aseptic powder injection of the composition containing the oxiracetam compound.
[0053] Accurately weigh the oxiracetam compound and mannitol according to the prescription of the fifth group in Table 1, add 1500ml of water for in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com