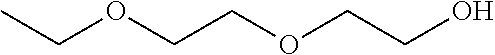
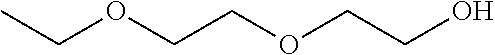
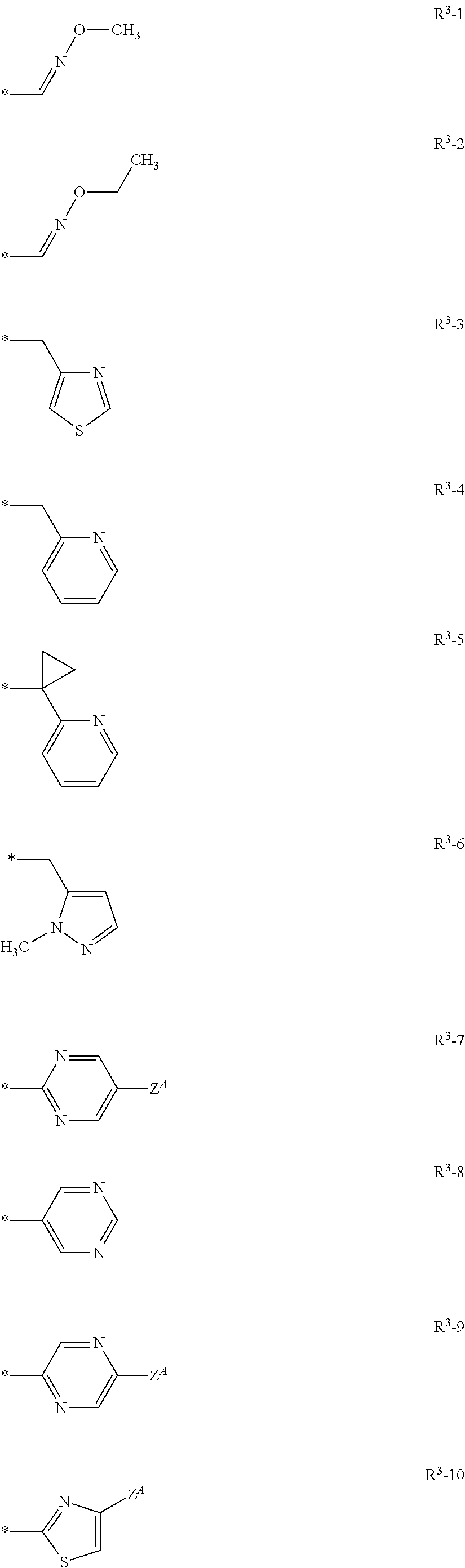
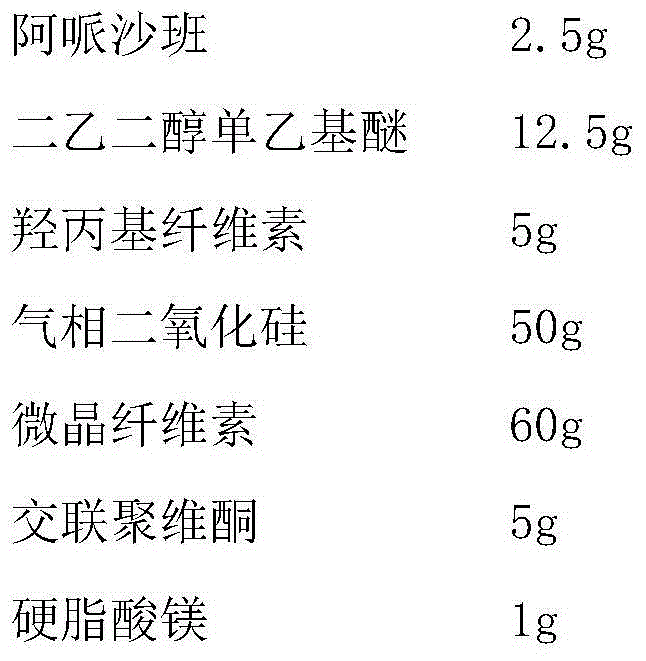
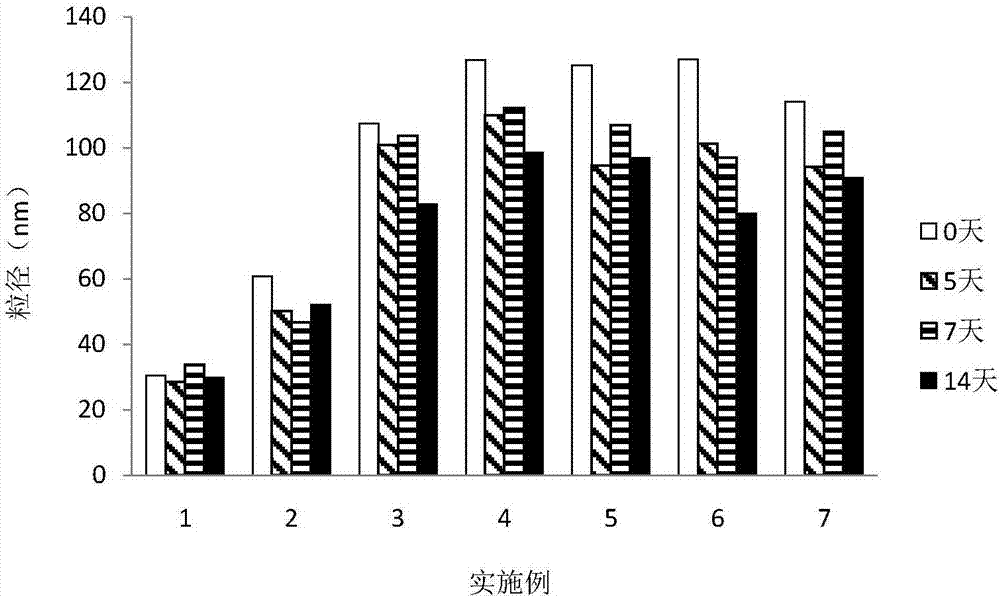
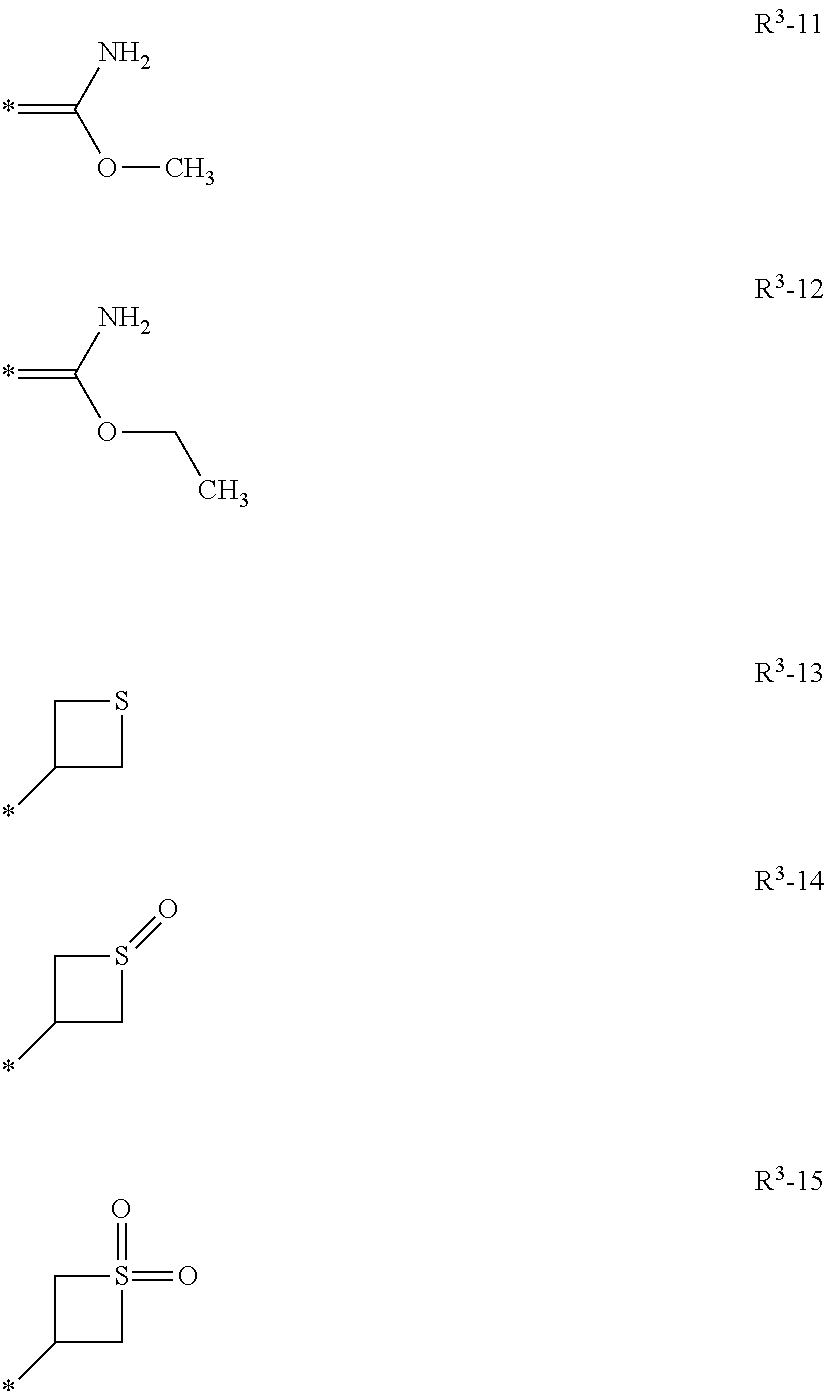Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
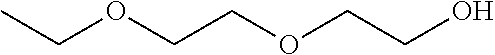
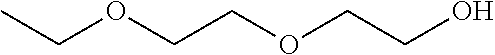
156 results about "Diethylene glycol monoethyl ether" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
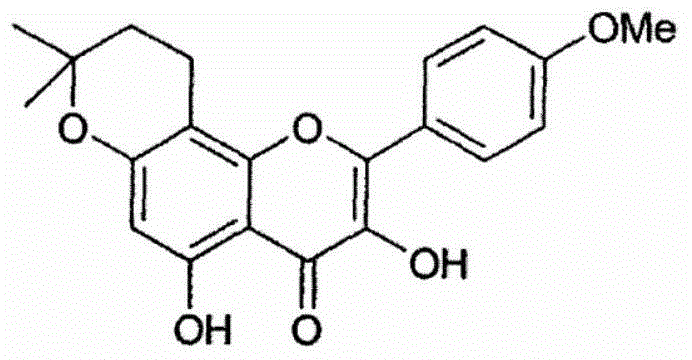
Compositions of pharmaceutical actives containing diethylene glycol monoethyl ether or other alkyl derivatives
InactiveUS20140296191A1Less viscousLess denseBiocideOrganic chemistryDiethylene glycol monoethyl etherNasal spray
The present invention relates to pharmaceutical compositions of various pharmaceutical actives, especially lyophilic and hydrophilic actives containing Diethylene glycol monoethyl ether or other alkyl derivatives thereof as a primary vehicle and / or to pharmaceutical compositions utilizing Diethylene glycol monoethyl ether or other alkyl derivatives thereof as a primary vehicle or as a solvent system in preparation of such pharmaceutical compositions. The pharmaceutical compositions of the present invention are safe, non-toxic, exhibits enhanced physical stability compared to conventional formulations containing such pharmaceutical actives and are suitable for use as injectables for intravenous and intramuscular administration, as well as for use as a preformed solution / liquid for filling in and preparation of capsules, tablets, nasal sprays, gargles, dermal applications, gels, topicals, liquid oral dosage forms and other dosage forms.
Owner:THEMIS MEDICARE LTD
Compositions of pharmaceutical actives containing diethylene glycol monoethyl ether or other alkyl derivatives
ActiveUS20180071390A1Less viscousLess denseOrganic active ingredientsAerosol deliveryUse medicationDiethylene glycol monoethyl ether
The present invention relates to pharmaceutical compositions of various pharmaceutical actives, especially lyophilic and hydrophilic actives containing Diethylene glycol monoethyl ether or other alkyl derivatives thereof as a primary vehicle and / or to pharmaceutical compositions utilizing Diethylene glycol monoethyl ether or other alkyl derivatives thereof as a primary vehicle or as a solvent system in preparation of such pharmaceutical compositions. The pharmaceutical compositions of the present invention are safe, non-toxic, exhibits enhanced physical stability compared to conventional formulations containing such pharmaceutical actives and are suitable for use as injectables for intravenous and intramuscular administration, as well as for use as a preformed solution / liquid for filling in and preparation of capsules, tablets, nasal sprays, gargles, dermal applications, gels, topicals, liquid oral dosage forms and other dosage forms.
Owner:THEMIS MEDICARE LTD
Pharmaceutical liquid composition containing pyridone derivative
A pharmaceutical liquid composition containing the Pirfenidone in a very high concentration of more or less 25% by weight can be obtained by dissolving the Pirfenidone in diethylene glycol monoethyl ether. Even when the liquid medicinal compositions are stored for a long period of time, the Pirfenidone will not be recrystallized with a good chemical and physical stability. Furthermore, the liquid compositions are little irritating to the wounds on the mucous membrane of the skin and suitable for the manufacture of pharmaceutical formulations to be administered either via the oral, percutaneous, nasal or vaginal routes or by means of spray, patch, inhalation, injection or intravenous drip.
Owner:KDL
Azilsartan tablet
ActiveCN104523632ADissolution rate is fastSimple processOrganic active ingredientsPill deliveryCelluloseDiethylene glycol monoethyl ether
The invention belongs to the technical field of medicines, and particularly relates to an azilsartan tablet. The azilsartan tablet contains azilsartan, hydroxy propyl cellulose and fumed silica, and is prepared by the following steps: dissolving the azilsartan and the hydroxy propyl cellulose in diethylene glycol monoethyl ether, adding the fumed silica to adsorb, uniformly mixing with pharmaceutically acceptable auxiliary materials and pressing by a direct tableting process. Compared with the prior art, the azilsartan tablet is high in drug dissolution speed and simple in process.
Owner:SHANDONG NEWTIME PHARMA
Liquid pest control formulation
ActiveUS20110086890A1Reduce stimulationIncrease percentageBiocideEther/acetal active ingredientsTocopheryl nicotinateDiethylene glycol monoethyl ether
The present invention relates to a liquid pest control system that includes a synthetic pyrethroid as a pest control active ingredient and an agent selected from the group consisting of purified diethylene glycol monoethyl ether, tocopherol nicotinate and tocopherol succinate, and combinations thereof, to reduce or eliminate paraesthesia of the synthetic pyrethroid. The system releases the synthetic pyrethroid efficiently and uniformly. The pest control system is less irritating to the animal's skin as compared to prior art systems, particularly to the small breeds of dogs. The system is useful for making liquid spot-on treatments, sprays and the like.
Owner:SERGEANTS PET CARE PRODS
Compositions of pharmaceutical actives containing diethylene glycol monoethyl ether or other alkyl derivatives
InactiveUS9827315B2Less viscousLess denseOrganic active ingredientsBiocideUse medicationDiethylene glycol monoethyl ether
The present invention relates to pharmaceutical compositions of various pharmaceutical actives, especially lyophilic and hydrophilic actives containing Diethylene glycol monoethyl ether or other alkyl derivatives thereof as a primary vehicle and / or to pharmaceutical compositions utilizing Diethylene glycol monoethyl ether or other alkyl derivatives thereof as a primary vehicle or as a solvent system in preparation of such pharmaceutical compositions. The pharmaceutical compositions of the present invention are safe, non-toxic, exhibits enhanced physical stability compared to conventional formulations containing such pharmaceutical actives and are suitable for use as injectables for intravenous and intramuscular administration, as well as for use as a preformed solution / liquid for filling in and preparation of capsules, tablets, nasal sprays, gargles, dermal applications, gels, topicals, liquid oral dosage forms and other dosage forms.
Owner:THEMIS MEDICARE LTD
Rivaroxaban tablet
ActiveCN104666262ADissolution rate is fastSimple processOrganic active ingredientsPill deliveryDiethylene glycol monoethyl etherMedicine
The invention belongs to the technical field of drugs and in particular relates to a rivaroxaban tablet. The rivaroxaban tablet contains rivaroxaban, hydroxypropyl cellulose and fumed silica and is prepared by dissolving rivaroxaban and hydroxypropyl cellulose in diethylene glycol monoethyl ether, adding fumed silica for adsorption, then mixing the materials with pharmaceutically acceptable auxiliary materials uniformly and pressing the mixture by adopting a direct tabletting process. Compared with the prior art, the rivaroxaban tablet is high in drug dissolution speed and simple in process and dispenses with addition of surfactants and micronization treatment.
Owner:SHANDONG NEWTIME PHARMA
Stable cefaclor tablet composition and preparation method thereof
ActiveCN103623412AImprove solubilityImprove bioavailabilityAntibacterial agentsOrganic active ingredientsDiethylene glycol monoethyl etherBioavailability
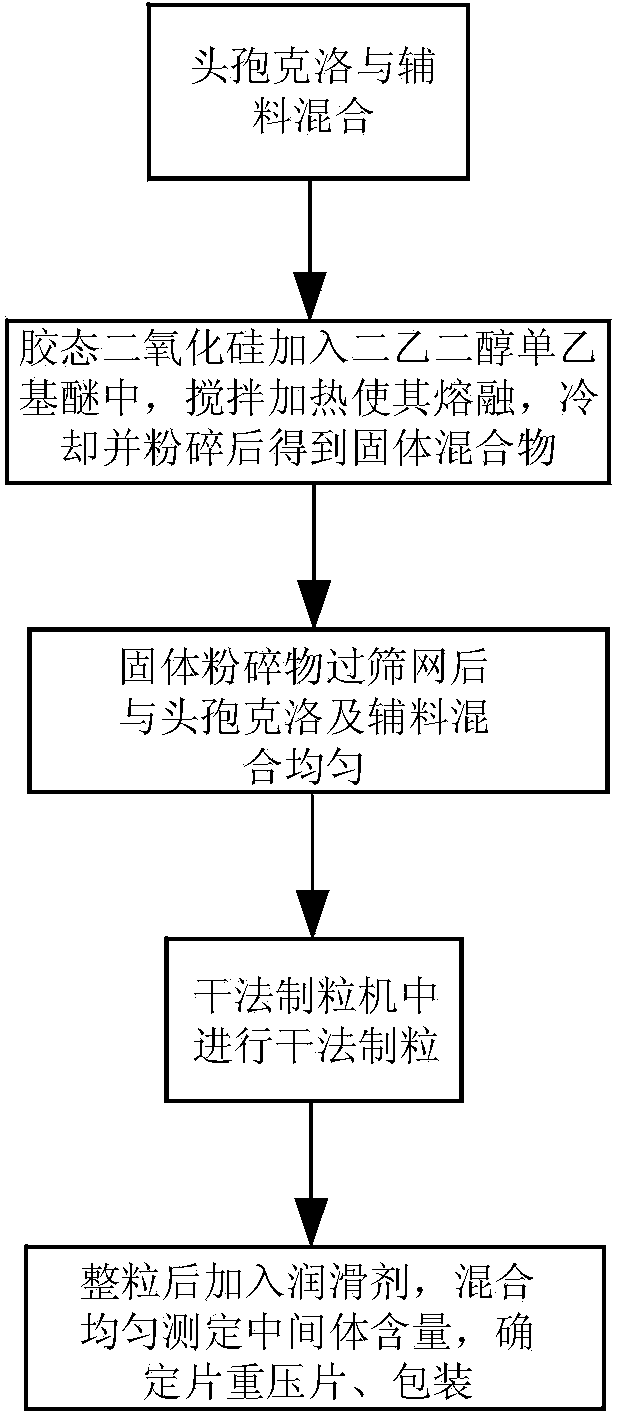
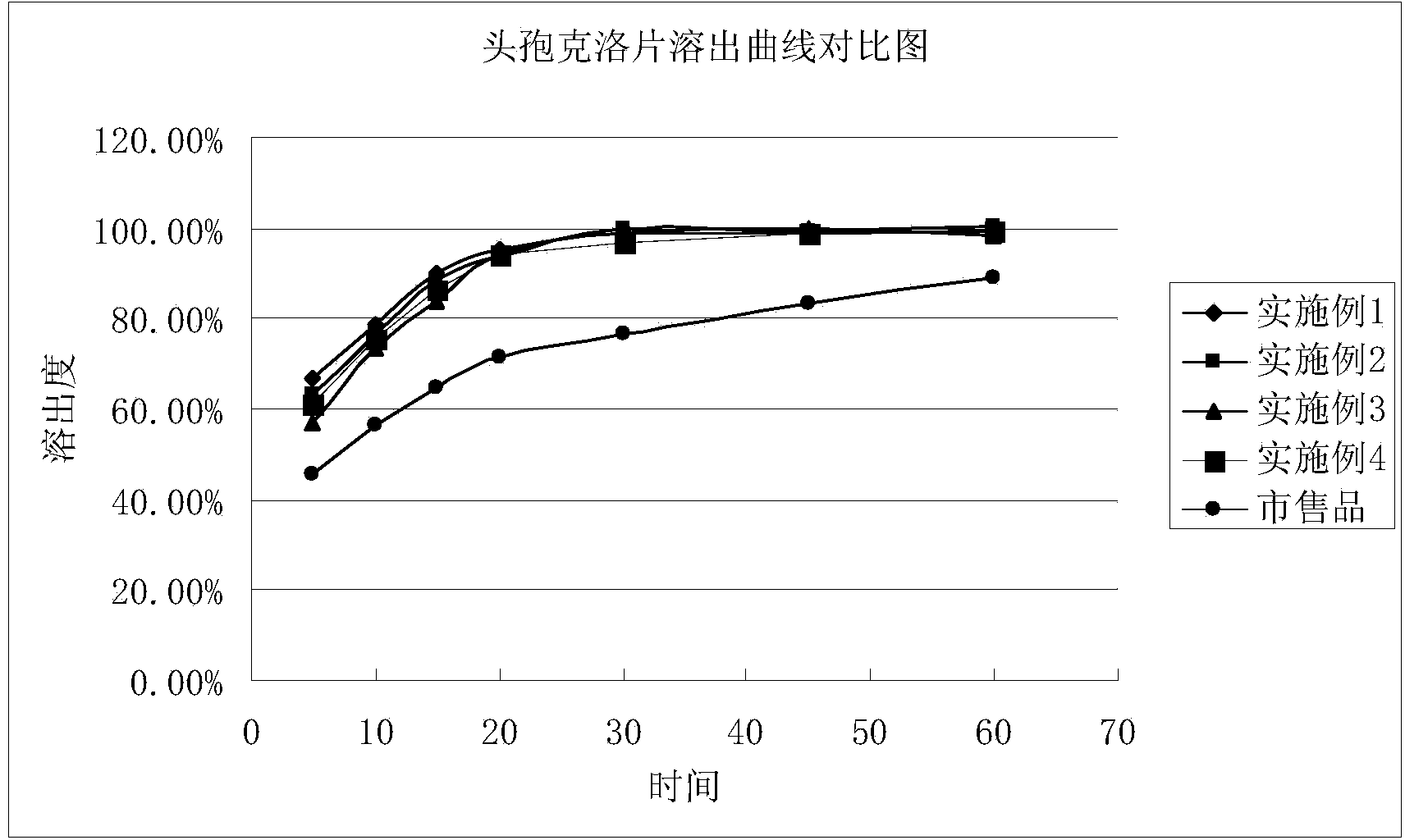
The invention discloses a cefaclor tablet pharmaceutical tablet composition which comprises cefaclor, lactose, microcrystalline cellulose, crosslinked povidone, diethylene glycol monoethyl ether, colloidal silicon dioxide and glyceryl behenate or sodium stearyl fumarate. The preparation method comprises the following steps: evenly mixing the cefaclor with the pharmaceutical auxiliary materials; adding the colloidal silicon dioxide into the diethylene glycol monoethyl ether, heating while stirring for melting, and cooling to obtain a solid mixture; screening, and evenly mixing the cefaclor auxiliary materials; granulating by a dry process; and after finishing the granules, adding the lubricant, evenly mixing, measuring the intermediate content, determining the tablet weight, tabletting and packaging. The invention provides a legal and reasonable cefaclor table composition and a preparation method thereof, thereby obtaining the cefaclor tablet which has the advantages of stabler and more controllable quality, higher bioavailability and simpler technique.
Owner:SHANGHAI NEW ASIATIC PHARMA MINHANG
Drug formulation containing a solubilizer for enhancing solubility, absorption, and permeability
InactiveUS20070021325A1Promote absorptionImprove permeabilityOrganic active ingredientsPeptide/protein ingredientsSolubilityBenzoic acid
Solubility, absorption, and permeability of drugs upon oral administration are improved when the drugs are mixed and / or complexed with water-miscible organic solvents. Illustratively, the absorption of a heparin-deoxycholic acid conjugate upon oral administration is increased by mixing and / or complexing this conjugate with dimethyl sulfoxide. Other illustrative water-miscible organic solvents include N-methylpyrrolidone, polyoxyl 35 castor oil, diethylene glycol monoethyl ether, and benzoic acid.
Owner:MEDIPLEX CORP
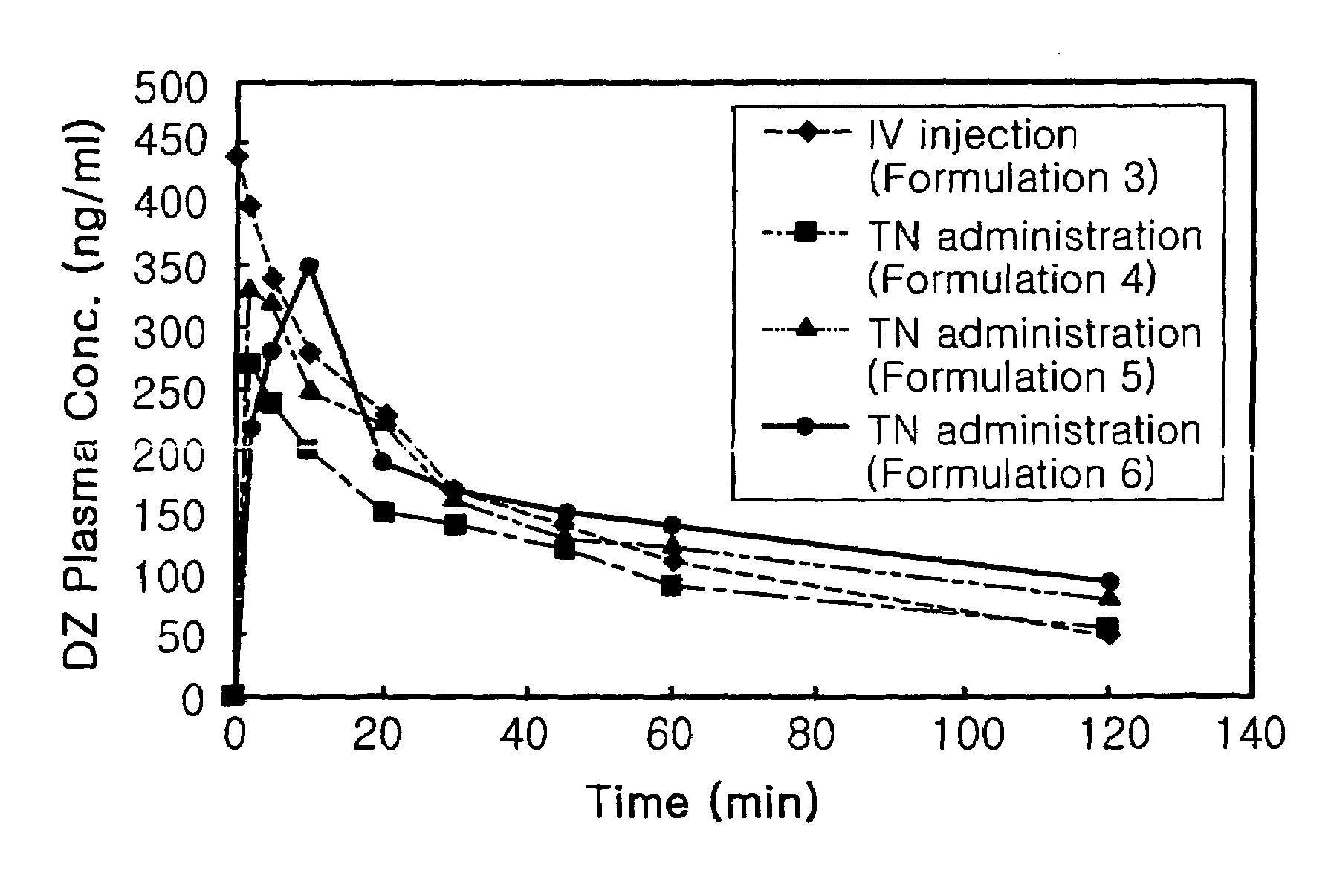
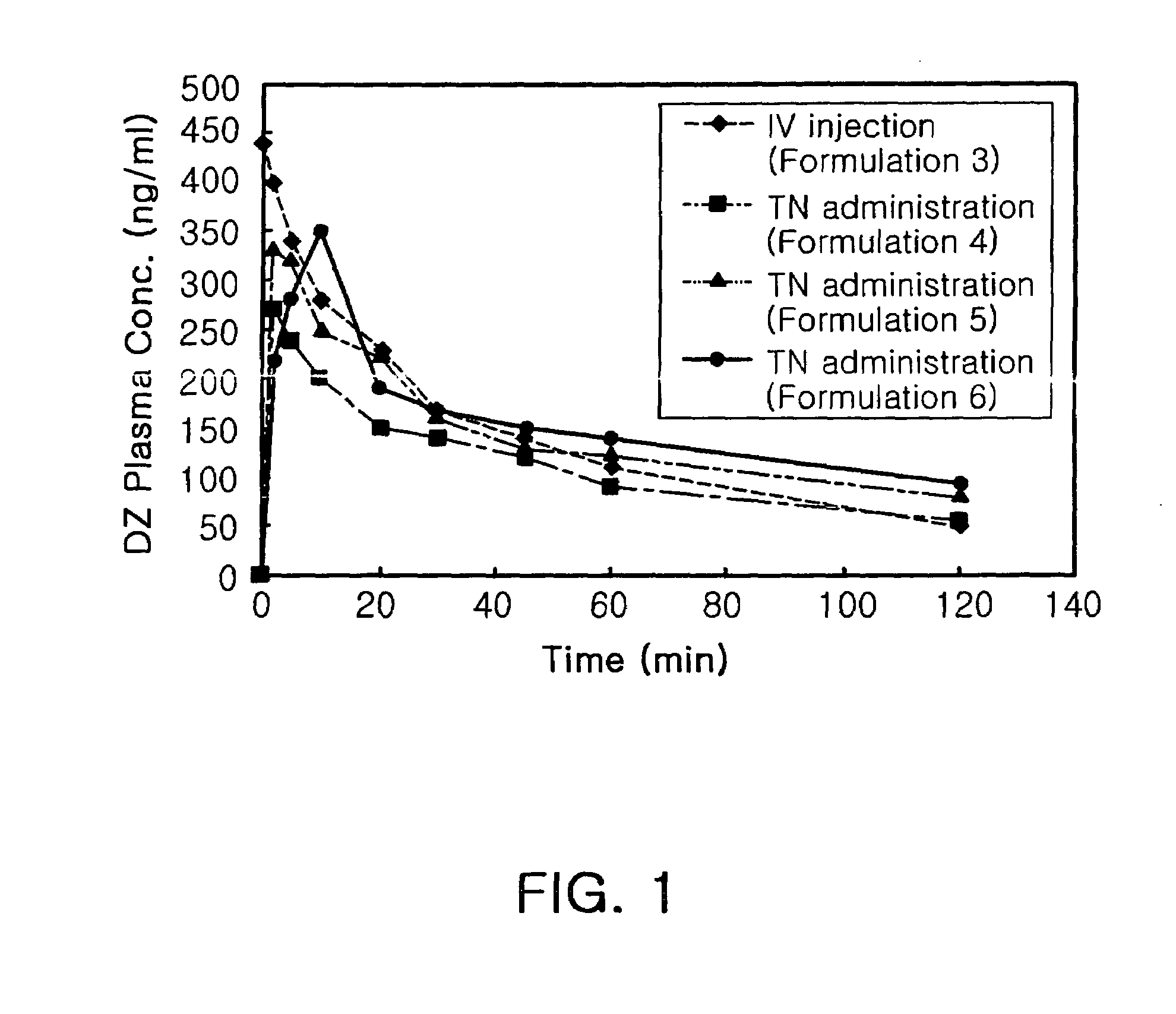
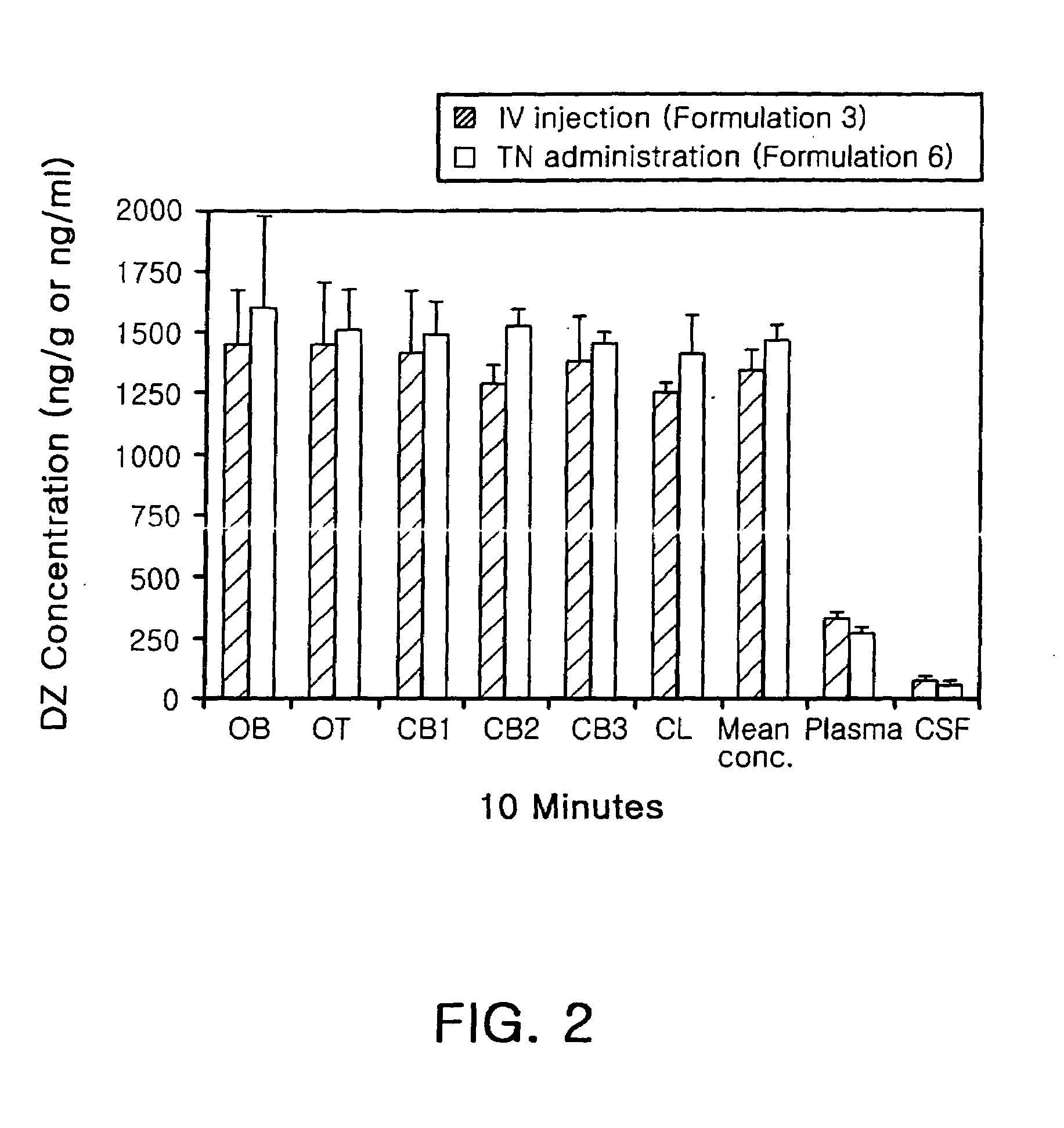
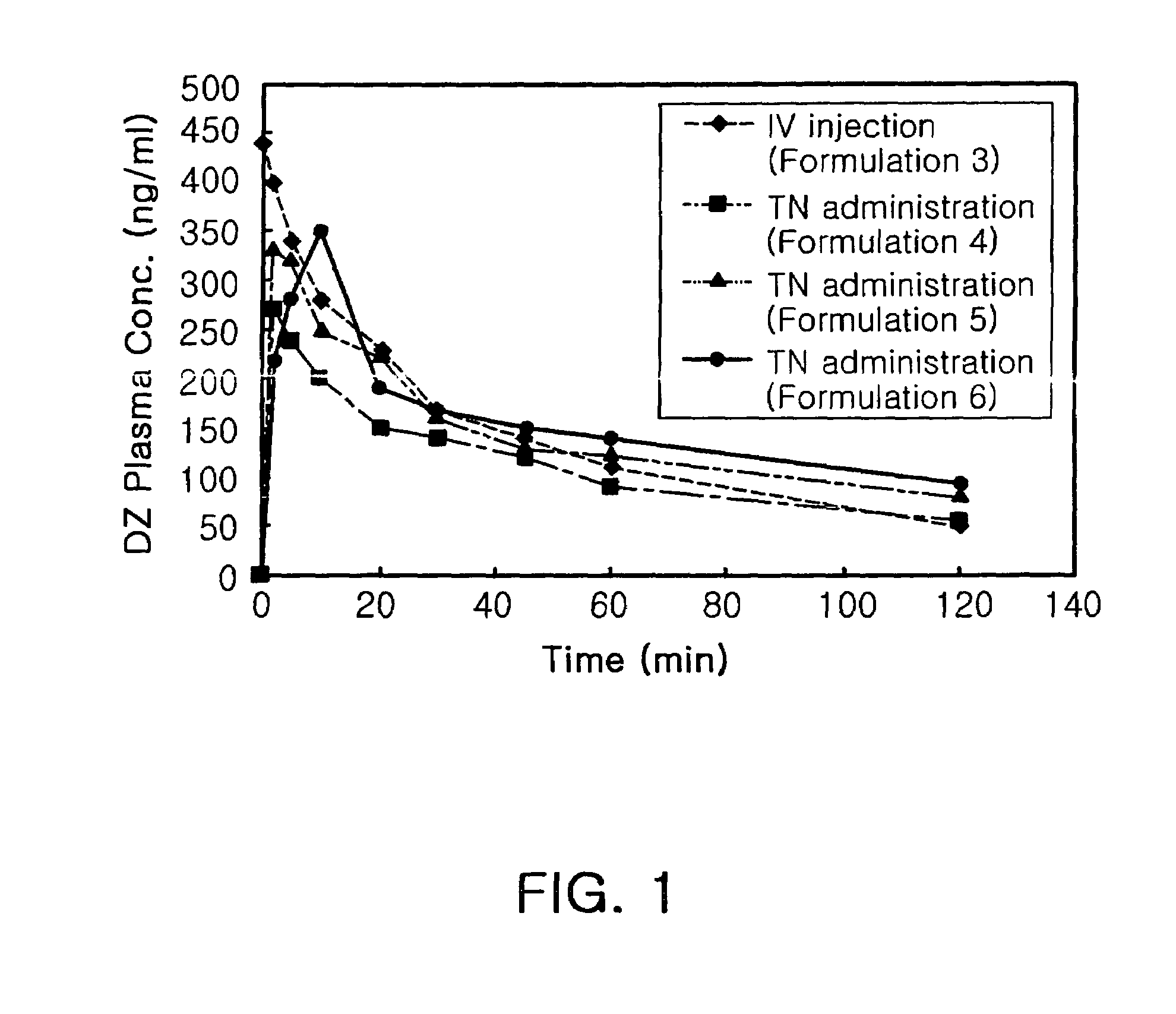
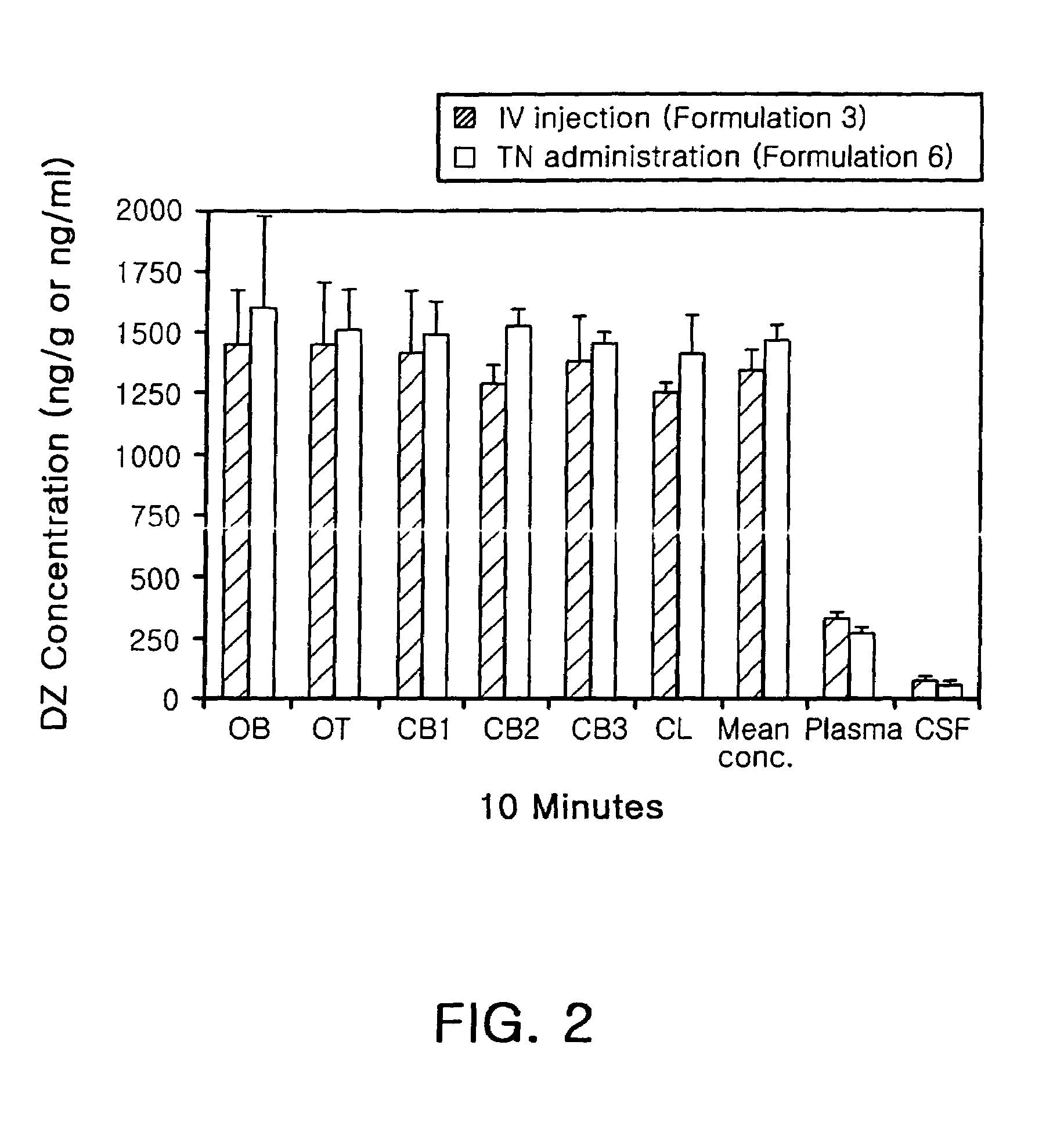
Transnasal anticonvulsive pharmaceutical composition
InactiveUS20080113970A1Improve permeabilityImprove solubilityBiocideNervous disorderConvulsionSolubility
Disclosed herein is a transnasal anticonvulsive pharmaceutical composition comprising diazepam as an active ingredient, water, a fatty acid ester, diethylene glycol monoethyl ether, ethanol and sodium glycocholate, wherein the weight of the fatty acid ester is at least 2-fold higher than that of water and is at least 2-fold higher than that of ethanol.The anticonvulsive pharmaceutical composition for transmucosal delivery of diazepam according to the present invention includes a minimized content of water and ethanol, a fatty acid ester as a main ingredient and no use of a polar solvent, e.g. glycol, and, exhibits improved diazepam solubility and transmucosal permeability due to using a small amount of water and ethanol. The present invention also includes treatment of convulsions by transnasally administering to a patient in need thereof a therapeutically effective amount of the disclosed compositions.
Owner:BIOPHARM
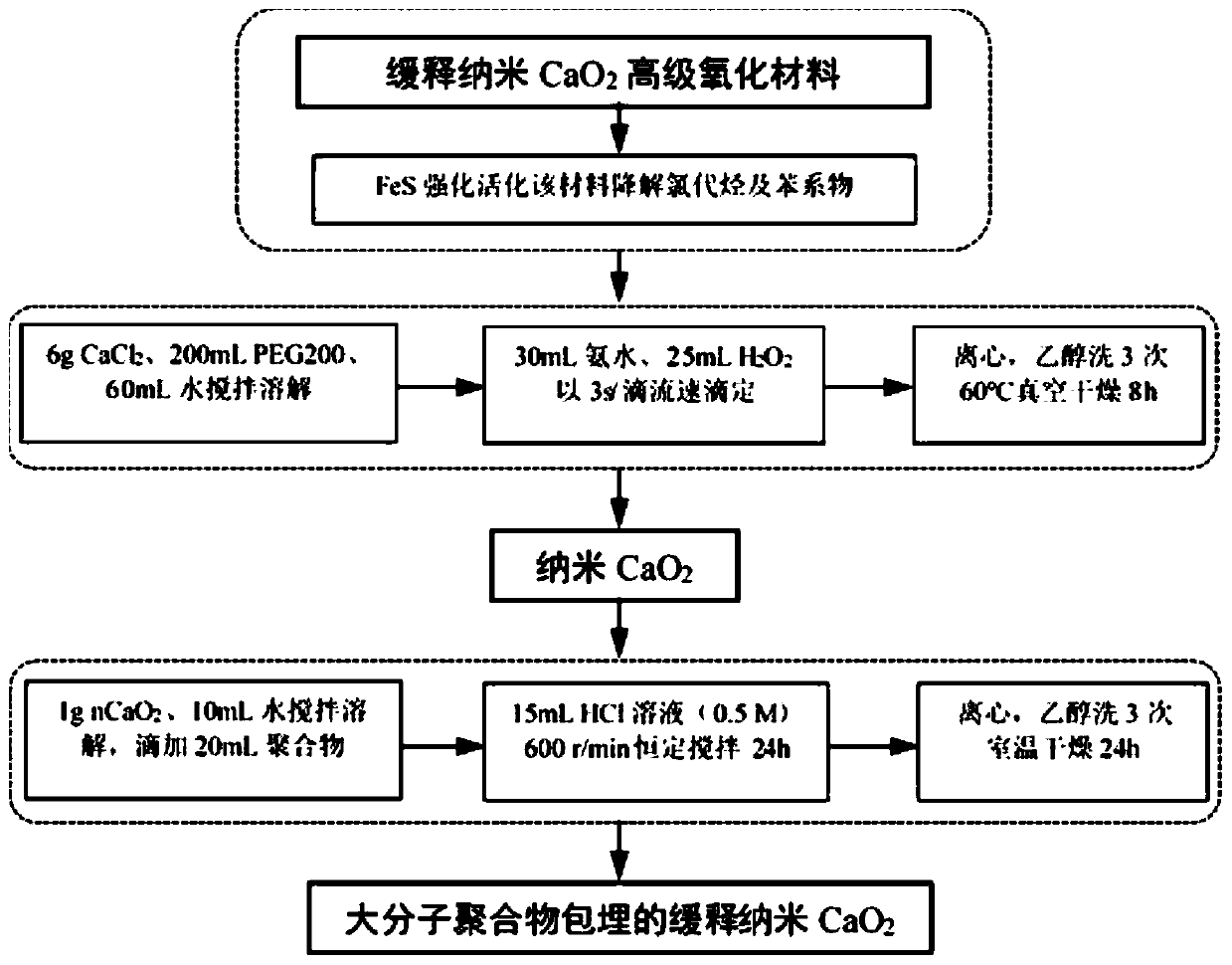
Sustained-release nano-calcium peroxide materials, preparation thereof, and method for removing chlorohydrocarbon and/or benzene series from underground water through sustained-release nano-calcium peroxide materials
PendingCN110759319AReduce manufacturing costEasy to operateMaterial nanotechnologyWater treatment compoundsPolyvinyl alcoholPolyethylene glycol
The invention belongs to the technical field of water treatment, and particularly relates to sustained-release nano-calcium peroxide materials, preparation thereof, and a method for removing chlorohydrocarbon and benzene series from underground water by strengthening and activating the sustained-release nano-calcium peroxide materials through ferrous sulfide. By taking calcium chloride, hydrogen peroxide with the mass fraction being 30% and ammonium hydroxide with the mass fraction being 30% as raw materials and by adding different dispersing agents, an irreversible coagulation phenomenon is avoided in the synthesis process, then by adding different macromolecular polymers such as polyethylene glycol 400 (PEG400), polyvinyl alcohol (PVA) and diethylene glycol monoethyl ether (DEGMME), a film is formed on the surface of nCaO2, and through processes such as washing with water and ethyl alcohol and vacuum drying, the sustained-release nano-calcium peroxide advanced oxidation materials embedded with the various macromolecular polymers are obtained. By applying the oxidation materials, the purposes of efficient and lasting treatment of pollutants and lowering of the repair cost of the underground water in contaminated sites are achieved.
Owner:EAST CHINA UNIV OF SCI & TECH
Anhydroicaritin tablet
ActiveCN105982869ADissolution rate is fastSimple processOrganic active ingredientsSkeletal disorderCelluloseDiethylene glycol monoethyl ether
The invention belongs to the technical field of medicines, and in particular relates to an anhydroicaritin tablet. The anhydroicaritin tablet contains anhydroicaritin, hydroxy propyl cellulose, fumed silica, diethylene glycol monoethyl ether and other pharmaceutically acceptable auxiliary materials. A preparation method comprises the following steps: dissolving anhydroicaritin and hydroxy propyl cellulose in diethylene glycol monoethyl ether, adding with fumed silica for adsorption, then uniformly mixing with the pharmaceutically acceptable auxiliary materials, and carrying out pressing by adopting a direct tableting technology. The medicine dissolution speed is high, the process is simple, a surfactant does not need to be added, and the micronization treatment is not needed. The acceleration test result shows that the prepared anhydroicaritin tablet is good in stability.
Owner:SHANDONG NEWTIME PHARMA
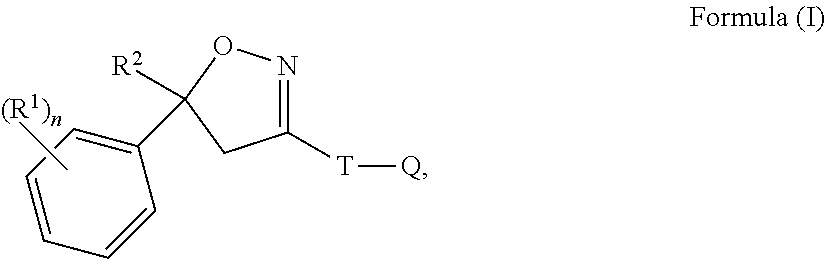
Isoxazoline compositions and use thereof in the prevention or treatment of parasite infestations in animals
This invention is directed to a pharmaceutical composition for drinking water administration comprising isoxazoline compounds of formula (I) and a polysorbate surfactant and diethylene glycol monoethyl ether (transcutol); and the use of the composition to treat or prevent parasite infestations of animals.
Owner:INTERVET INC
Apixaban tablet
ActiveCN105982870ADissolution rate is fastSimple processOrganic active ingredientsPharmaceutical non-active ingredientsCelluloseDiethylene glycol monoethyl ether
The invention belongs to the technical field of pharmaceutical preparations, and in particular relates to an apixaban tablet. The apixaban tablet contains apixaban, hydroxy propyl cellulose and fumed silica. A preparation method of the apixaban tablet comprises the following steps: dissolving apixaban and hydroxy propyl cellulose in diethylene glycol monoethyl ether, adding with fumed silica for adsorption, then uniformly mixing with pharmaceutically acceptable auxiliary materials, and carrying out pressing by adopting a direct tableting technology. Compared with the prior art, the apixaban tablet has the advantages that the medicine dissolution speed is high, the process is simple, a surfactant does not need to be added, and the micronization treatment is not needed. The acceleration test result shows that the prepared apixaban tablet is good in stability.
Owner:SHANDONG NEWTIME PHARMA
Ezetimibe tablet
ActiveCN104666260ADissolution rate is fastSimple processOrganic active ingredientsMetabolism disorderDiethylene glycol monoethyl etherFumed silica
The invention belongs to the technical field of drugs and in particular relates to an ezetimibe tablet. The ezetimibe tablet comprises ezetimibe, hydroxypropyl cellulose, diethylene glycol monoethyl ether, fumed silica, filling agents, disintegrating agents and lubricating agents. The tablet is prepared by dissolving ezetimibe and hydroxypropyl cellulose in diethylene glycol monoethyl ether, adding fumed silica for adsorption, then mixing the materials with pharmaceutically acceptable auxiliary materials uniformly and pressing the mixture by adopting a direct tabletting process. Compared with the prior art, the ezetimibe tablet is high in drug dissolution speed and simple in process and dispenses with addition of surfactants and micronization treatment.
Owner:SHANDONG NEWTIME PHARMA
Photoresist developer compositions
ActiveCN1503064AGood compatibilitySemiconductor/solid-state device manufacturingPhotosensitive material processingDiacetone alcoholSodium bicarbonate
A photoresist developer composition is provided, to improve miscibility and to suppress or minimize the generation of residue after development, thereby enhancing the precision of photoresist pattern. The photoresist developer composition comprises 1-10 wt% of an inorganic alkali; 0.1-3.0 wt% of an organic solvent; 1.0-20.0 wt% of a surfactant; and 67-97.9 wt% of water. Preferably the inorganic alkali is selected from the group consisting of KOH, NaOH, sodium phosphate, sodium silicate, sodium carbonate, sodium bicarbonate and their mixtures; the organic solvent is selected from the group consisting of methanol, ethanol, 1-propanol, 2-propanol, butanol, diacetone alcohol, ethylene glycol monomethyl ether, ethylene glycol monoethyl ether, ethylene glycol monobutyl ether, diethylene glycol monomethyl ether, diethylene glycol monoethyl ether, dipropyl glycol monomethyl ether, dipropyl glycol monoethyl ether and their mixtures; and the surfactant is a mixture of a nonionic surfactant and an anionic surfactant.
Owner:DONGJIN SEMICHEM CO LTD
Dustproof and bactericidal type aqueous coating material for glass doors and preparation method thereof
InactiveCN104342003AImprove the bactericidal effectImprove dustproofPaints with biocidesPolyurea/polyurethane coatingsPolymer sciencePolyvinyl alcohol
The invention discloses a dustproof and bactericidal type aqueous coating material for glass doors. The dustproof and bactericidal type aqueous coating material is characterized by being prepared from the following raw materials in parts by weight: 34-38 parts of aqueous polyurethane resin, 28-34 parts of acrylic epoxy resin, 8-12 parts of modified styrene-acrylic emulsion, 0.4-0.8 part of polyvinyl alcohol, 1-3 parts of diethylene glycol monoethyl ether, 0.3-0.6 part of alcohol-amine dipyrophosphate acyloxy glycolate titanate, 0.2-0.5 part of polyether modified polyorganosiloxane, 0.2-0.4 part of cellaburate, 0.3-0.6 part of dioctyl sodium sulfosuccinate, 0.4-0.7 part of acetoacetoxy ethyl methacrylate, 0.6-1.2 parts of climbazole, 3-5 parts of ethylene glycol, 0.5-1.0 part of graphene, 1-2 parts of pigment and 10-15 parts of deionized water. According to the aqueous coating material disclosed by the invention, on the basis of high adhesion, water resistance and alcohol acid resistance, the modified styrene-acrylic emulsion is added, so that the bactericidal, dustproof and anti-electrostatic effects of the coating material are improved; added climbazole has spectrum sterilization property, so that the quality of the coating material is further improved; and the coating material disclosed by the invention is simple in operation and low in production cost, does not contain benzene solvents and heavy metals, is harmless to human bodies and environment, is environment-friendly and safe and is suitable for being popularized.
Owner:凤阳徽亨商贸有限公司
Ultrasonic nano spray liquid preparation and preparation method thereof
InactiveCN107496196APromote absorptionTo promote metabolismCosmetic preparationsToilet preparationsPolyethylene glycolOil phase
The invention relates to an ultrasonic nano spray liquid preparation, which is prepared from the following components in parts by weight: 1-10 parts of coenzyme Q10, 1-100 parts of hyaluronic acid, 1-20 parts of an oil phase material, 2-20 parts of an emulsifier, 1-100 parts of a co-emulsifier and 100-10000 parts of ethanol and / or water, wherein the oil phase material is one or a combination of more of medium chain triglyceride (MCT), vitamin E and soybean oil; the emulsifier is one or a combination of more of castor oil polyoxyethylene ether, hydrogenated castor oil poly(hydrocarbon oxygen ester) 40 and Tween-80; the co-emulsifier is one or a combination of more of diethylene glycol monoethyl ether, labrasol and caprylic capric triglyceride. The invention also provides a preparation method of the ultrasonic nano spray liquid preparation; the method comprises the following steps: evenly mixing the oil phase material, the emulsifier, the co-emulsifier and the coenzyme Q10 so as to obtain a mixed oil phase; dissolving hyaluronic acid into a solvent to obtain a water phase; dispersing the mixed oil phase into the water phase, and carrying out ultrasonic dispersion. The ultrasonic nano spray liquid preparation is stable in performance; nanoscale fine fogdrops are enabled to permeate the skin deep in a form of ultrasonic spray, so that the skin absorption of the coenzyme Q 10 and the hyaluronic acid is improved.
Owner:SUZHOU UNIV
Nucleic acid releasing agent and HPV virus nucleic acid detection kit
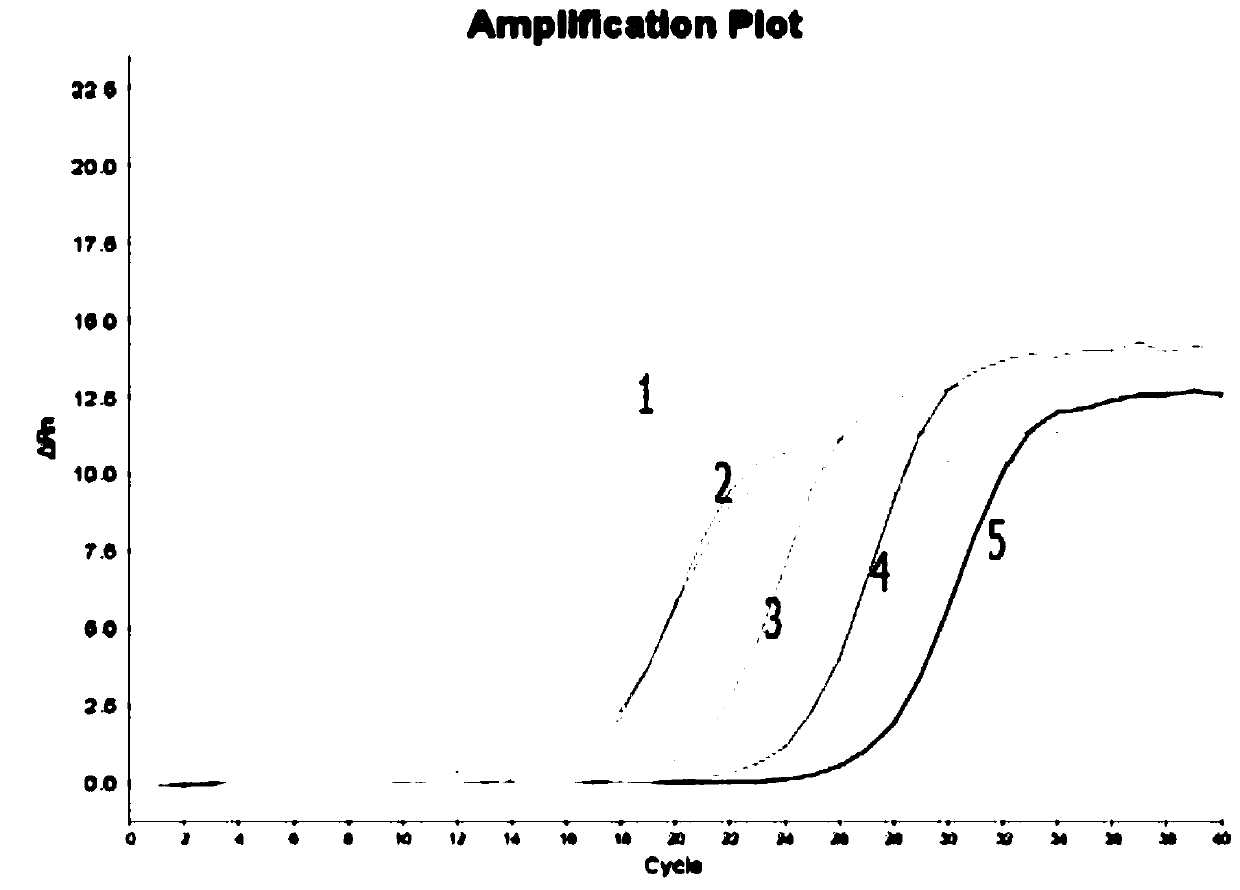
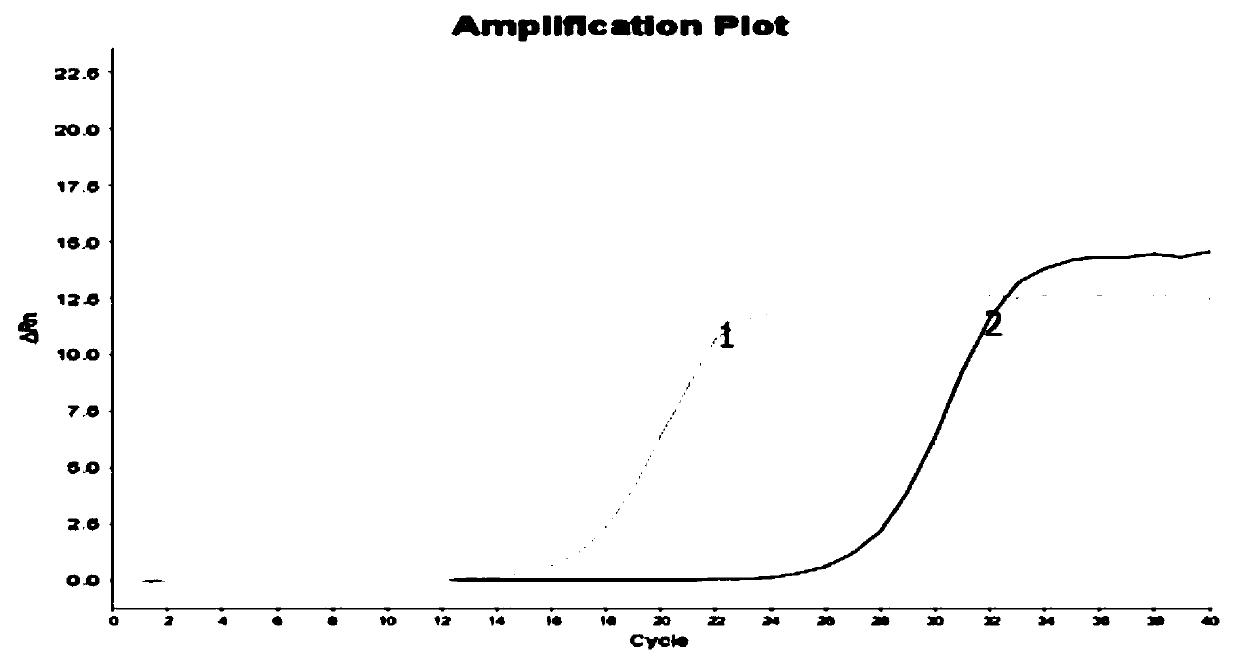
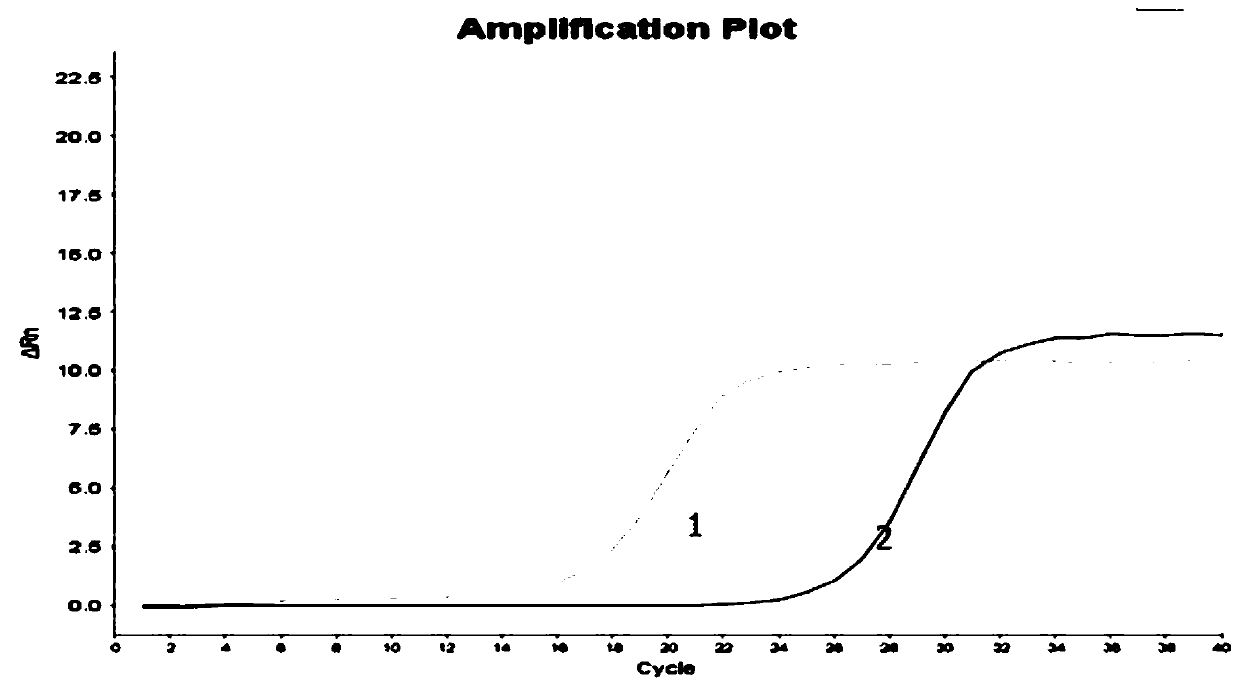
PendingCN111172240AAct quicklySubsequent experiment impactMicrobiological testing/measurementDiethylene glycol monoethyl etherNucleic acid detection
The invention discloses a nucleic acid releasing agent. The nucleic acid releasing agent comprises a composition of Triton X-100 and diethylene glycol monoethyl ether (DCDE) with a mass ratio of 1: (1-3). The low-cost composition of Triton X-100 and DCDE is adopted to replace a traditional guanidine salt and a detergent, and the obtained nucleic acid releasing agent has advantages of safe use, easy degradation, simple operation, and low manufacturing cost, can effectively lyse serum samples containing pathogens, quickly release nucleic acids therein, reduce inhibition of downstream experiments, and has high pathogen lysis efficiency. The invention also discloses an HPV virus nucleic acid detection kit. The kit comprises the nucleic acid releasing agent, is simple and convenient to operate,and high in working efficiency, effectively avoids a link that may occur during the experimental operation, reduces loss of HPV DNA in an extraction process, and has a quantification limit of 45 IU / mL and a detection limit of 22 IU / mL.
Owner:苏州博方生物技术有限公司
Transnasal anticonvulsive pharmaceutical composition
InactiveUS7745430B2Improve permeabilityImprove solubilityBiocideNervous disorderSolubilityConvulsion
Owner:BIOPHARM
Stripping liquid composition for photoresist
InactiveCN1402089AAggressiveInvasiveness does not produceSemiconductor/solid-state device manufacturingPhotosensitive material processingSolubilityEthylene glycol monophenyl ether
Provided is an exfoliating solution composition for photoresist, which is excellent in solubility and exfoliating property and has the low corrosiveness against metal wires and minimizes the impregnation into O-rings and pipe lines and has low volatility and low toxicity which minimizes the environmental pollution and the toxicity problems for the operators. The exfoliating solution composition comprises 10-40wt% of an organic amine compound and 10-80wt% of at least one protogenic polar solvent selected from the group consisting of ethylene glycol monomethyl ether, ethylene glycol monoethyl ether, ethylene glycol monobutyl ether, ethylene glycol monophenyl ether, butyl carbitol, diethylene glycol monoethyl ether, and etc., wherein the composition always contains at least 5-30wt% of dipropylene glycol monomethyl ether and / or dipropylene glycol monomethyl ether acetate as a low toxic / hydrophilic polar solvent in the polar solvent.
Owner:DUKSUNG
Rust preventive oil and preparation method thereof
The invention relates to the field of ferrous metal and copper alloy sealing storage and corrosion prevention, and particularly relates to rust preventive oil and a preparation method thereof. The rust preventive oil consists of the following raw materials in parts by weight: 62-65 parts of kerosene, 5-6 parts of zinc naphthenate, 2-3 parts of triethoxyvinylsilane, 2-3 parts of dodecenylsuccinic acid, 3-5 parts of diethylene glycol monoethyl ether, 0.2 part of Vaseline and 0.2 part of 2,6 butylated hydroxytoluene. The rust preventive oil plays an outstanding rust preventing role in storage and has an excellent rust preventing effect on cast iron, copper and the like; a workpiece can be stored for over two years in sealing by corrosion prevention; more importantly, the rust preventive oil neither discolors easily nor oxidizes easily; in addition, sealing removal is simple and convenient, the sealed rust preventive oil can be wiped away just by means of wetting spun cotton with a little kerosene or gasoline, and the appearance of the workpiece is not affected; the rust preventive oil is excellent in comprehensive properties and strong in universality.
Owner:李阳
Non-aqueous inkjet ink and ink set
Owner:GENERAL CO LTD
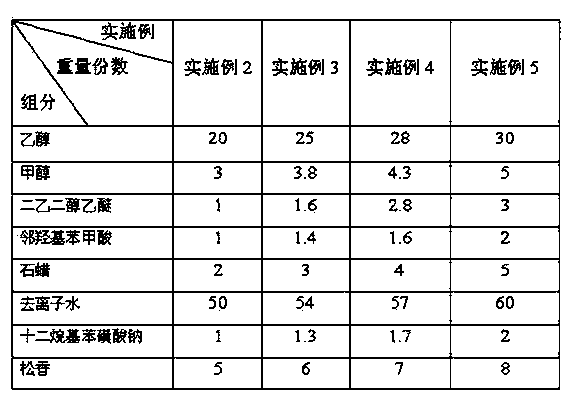
Traffic control circuit welding welding-assisting material and preparing method thereof
ActiveCN103831548AImprove anti-aging propertiesImprove high and low temperature resistanceWelding/cutting media/materialsSoldering mediaBenzoic acidDiethylene glycol monoethyl ether
The invention discloses a traffic control circuit welding welding-assisting material and a preparing method of the welding-assisting material and relates to the technical field of welding. The traffic control circuit welding welding-assisting material comprises, by weight, 20-30 parts of ethyl alcohol, 3-5 parts of methyl alcohol, 1-3 parts of diethylene glycol monoethyl ether, 1-2 parts of o-hydroxybenzoic acid, 2-5 parts of wax, 1-2 parts of emulgator, 50-60 parts of deionized water and 5-8 parts of colophony. The traffic control circuit welding welding-assisting material can be used for obviously improving the high-low temperature resisting feature and the welding strength of a welded finished product and meanwhile, can protect the health of an operating worker and the atmosphere environment.
Owner:河北雄业华阳交通科技有限公司
Topical roflumilast formulation having improved delivery and plasma half life
ActiveUS11129818B2Decrease therapeutic successIncreasing imperfectionAerosol deliveryOintment deliveryDiethylene glycol monoethyl etherBlood plasma
Owner:ARCUTIS BIOTHERAPEUTICS INC
Aprepitant capsules
ActiveCN104586814ADissolution rate is fastSimple processOrganic active ingredientsDigestive systemDiethylene glycol monoethyl etherDissolution
The invention belongs to the technical field of medicines, and specifically relates to aprepitant capsules. The aprepitant capsules contain aprepitant, hydroxy propyl cellulose, diethylene glycol monoethyl ether, fumed silica and other pharmaceutically acceptable filler, disintegrant and lubricant. A preparation method of the aprepitant capsules comprises the following steps: dissolving aprepitant in diethylene glycol monoethyl ether, adding hydroxy propyl cellulose, stirring to dissolve, adding fumed silica to adsorb, then uniformly mixing with the other pharmaceutically acceptable filler, disintegrant and lubricant, and directly filling capsules. Compared with the prior art, the aprepitant capsules disclosed by the invention are fast in medicine dissolution speed, simple in process, and free from a surfactant and a micronization treatment. An acceleration test result indicates that the prepared aprepitant capsules are high in dissolution rate.
Owner:SHANDONG NEWTIME PHARMA
Polypropylene waterproof gas-permeable film
The invention discloses a polypropylene waterproof gas-permeable film, which is prepared by carrying out a reaction on main raw materials comprising polypropylene, calcium carbonate and bentonite, glycerol ester of rosin and various auxiliary agents. According to the invention, a modifier is added into reactants and is used for modifying polypropylene, and modification comprises: carrying out a polymerization reaction at a temperature of 80-100 DEG C by using 4-fluorophthalic anhydride, diethylene glycol monoethyl ether and (1S,2S)-(+)-2-amino-1-(4-nitrophenyl)-1,3-propanediol as reactants; aregulating agent is added, is a mixture of 4,4'-diamino-2,2'-dinitrodiphenylmethane, dimethyl maleic anhydride and 1,2-epoxy-4-vinylcyclohexane, and can make the modifier be uniformly dispersed in polypropylene so as to substantially improve the modification effect of the modifier; and the finally prepared polypropylene waterproof gas-permeable film has characteristics of strong waterproof gas permeability, large moisture permeability, large tensile strength and large elongation at break.
Owner:湖北联利非织造布技术创新中心有限公司
Anhydroicaritin oral preparation and preparation method thereof
ActiveCN105412002AParticle size unchangedSimple preparation processOrganic active ingredientsOrganic non-active ingredientsDiethylene glycol monoethyl etherChemistry
The invention discloses an anhydroicaritin oral preparation and a preparation method thereof. The anhydroicaritin preparation contains diethylene glycol monoethyl ether and povidone. Compared with the prior art, liquid medicine is diluted into water, the solution is clear and transparent, the stability is good, the particle size range is 20-100 nm, the preparation technology is simple, and safety is high.
Owner:SHANDONG NEWTIME PHARMA
Pharmaceutical composition for topical administration and preparation method therefor
ActiveUS20200276109A1Organic active ingredientsOintment deliveryDiethylene glycol monoethyl etherPharmaceutical medicine
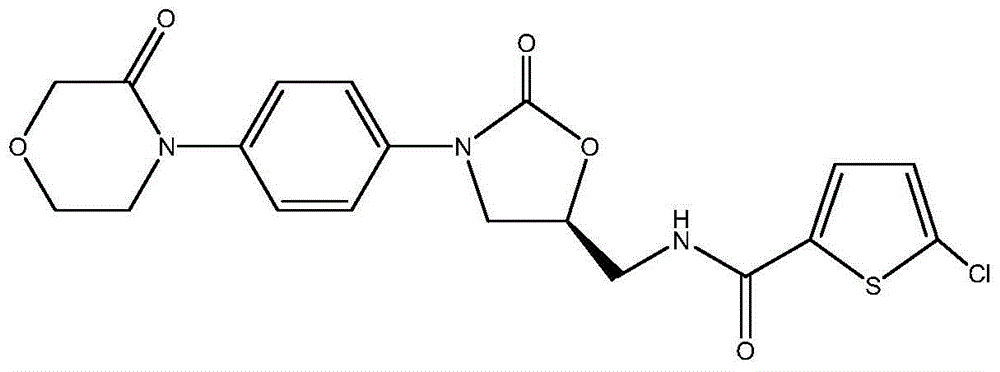
A pharmaceutical composition for topical administration and a preparation method therefor are described. In particular, a pharmaceutical composition comprising (3aR,5s,6aS)-N-(3-methoxy-1,2,4-thiadiazol-5-yl)-5-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)hexahydrocyclopenta[c]pyrrole-2(1H)-carboxamide, or a pharmaceutically acceptable salt thereof, and diethylene glycol monoethyl ether is described.
Owner:JIANGSU HENGRUI MEDICINE CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com