Compound repaglinide-metformin hydrochloride solid quick-release preparation and preparation method and application thereof
A technology of metformin hydrochloride and immediate-release preparations, which is applied in the field of medicine and can solve problems such as the increase of repaglinide substances
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
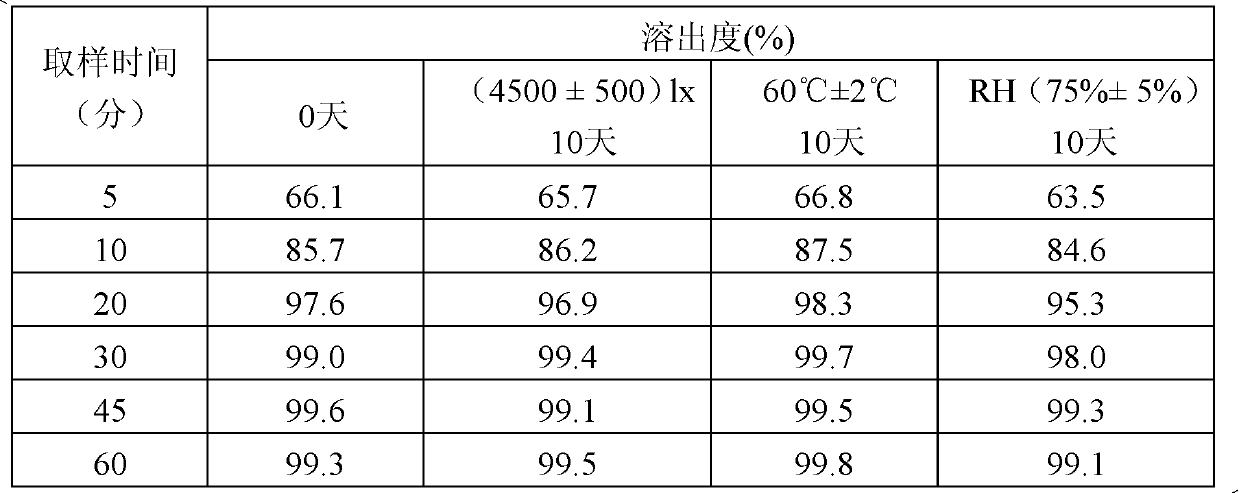
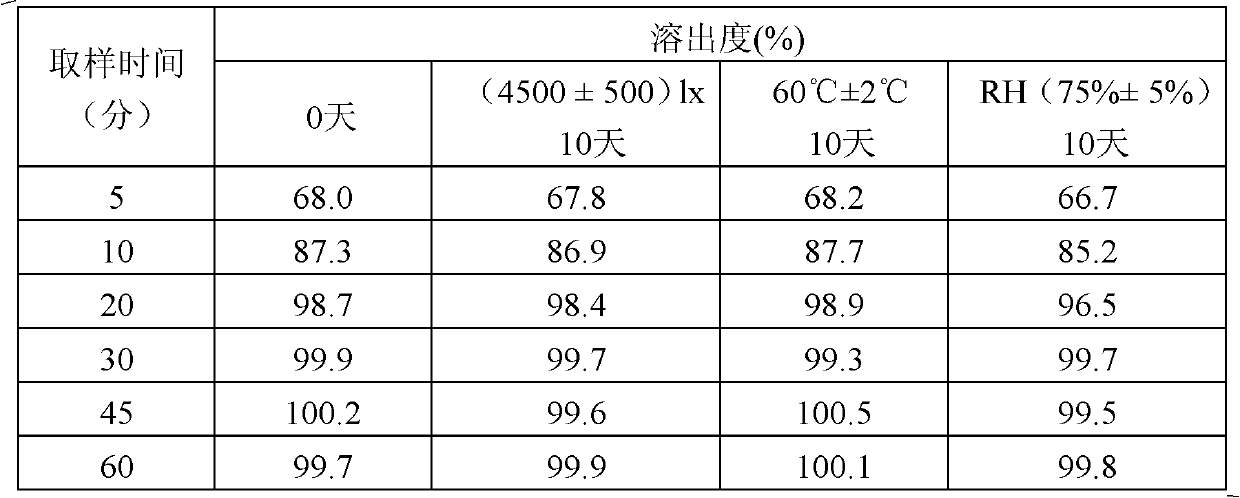
[0029] Investigation on the stability of influencing factors for immediate-release tablets obtained by two preparation processes
[0030] Compound repaglinide-metformin hydrochloride immediate-release tablets are prepared by two preparation processes, one is to mix repaglinide and metformin hydrochloride together with excipients for tablet compression, and the other is to mix repaglinide and metformin hydrochloride Metformin is separately mixed with auxiliary materials to make granules, and then the granules are mixed for tabletting.
[0031] Prescription of Compound Immediate Release Tablets Containing Repaglinide 1mg Metformin Hydrochloride 500mg (500 Tablets)
[0032]
[0033]Preparation method 1: Grind metformin hydrochloride and various auxiliary materials separately, and pass through an 80-mesh sieve. The repaglinide was pulverized and passed through a 250-mesh sieve. Take the pulverized main drug and lactose according to the prescription amount, weigh 5g of crospov...
Embodiment 2
[0049] Prescription of Compound Immediate Release Tablets Containing Repaglinide 2mg Metformin Hydrochloride 500mg (500 Tablets)
[0050] (1) Repaglinide Granules
[0051]
[0052] (2) Metformin Hydrochloride Granules
[0053]
[0054]
[0055] (3) Accessories
[0057] Crospovidone 4g
[0058] Preparation method: Grind metformin hydrochloride and various auxiliary materials separately, and pass through 80-mesh sieve. The repaglinide was pulverized and passed through a 250-mesh sieve. Weigh repaglinide, poloxamer 188, microcrystalline cellulose and crospovidone according to the prescription amount, and mix well; weigh 0.8g PVP K30 and add water to prepare a 25% solution, add the mixed Repaglinide and fine powder of auxiliary materials are used to make a suitable soft material; the prepared soft material is made into wet granules through a 40-mesh sieve, and the wet granules are dried at 40°C to obtain dry granules; the dry granules a...
Embodiment 3
[0060] Prescription of Compound Immediate Release Tablets Containing Repaglinide 2mg Metformin Hydrochloride 1000mg (500 Tablets)
[0061] (1) Repaglinide Granules
[0062]
[0063] (2) Metformin Hydrochloride Granules
[0064]
[0065]
[0066] (3) Accessories
[0067] Glyceryl Behenate 2.9g
[0068] Croscarmellose Sodium 6g
[0069] Preparation method: Grind metformin hydrochloride and various auxiliary materials separately, and pass through 80-mesh sieve. The repaglinide is pulverized by airflow, and the particle size of more than 90% of the pulverized powder is controlled to be below 20 μm. Weigh repaglinide, mannitol and croscarmellose sodium according to the prescription amount, and mix well; weigh 0.7g PVP K30 and add water to make a 25% solution, add the mixed repaglinide, auxiliary materials Fine powder, make suitable soft material; pass the prepared soft material through a 40-mesh sieve to make wet granules, dry the wet granules at 40°C to obtain dry gr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com