Repaglinide synthesis process
A process and compound technology, applied in the field of synthesizing repaglinide, can solve the problems of long reaction time, low yield, high price, etc., and achieve the effect of simple operation, easy-to-obtain raw materials, and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
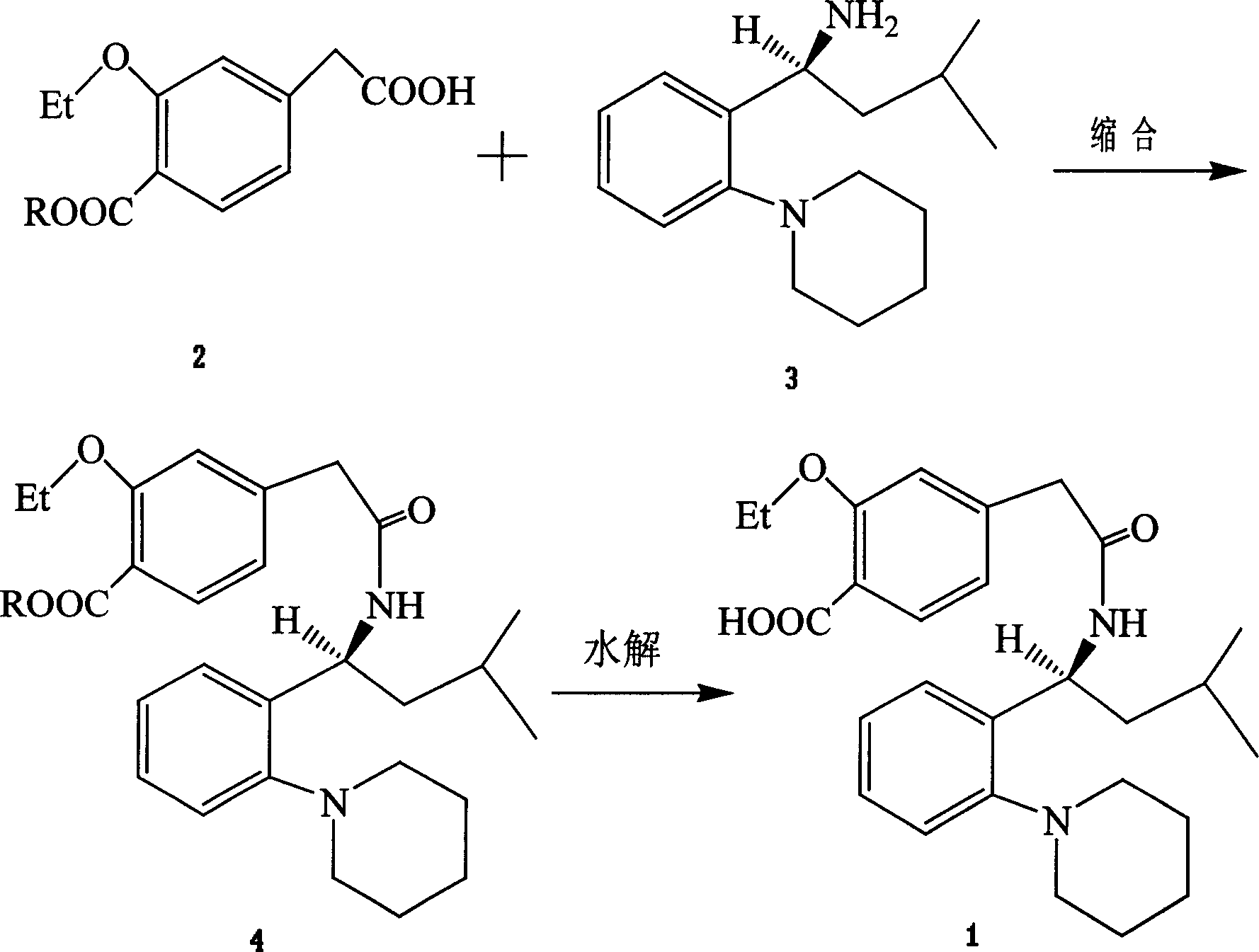
[0032] Ethyl S(+)-2-ethoxy-4-[N-{1-(2-piperidinylphenyl)-3-methyl-1-butyl}aminocarbonylmethyl]benzoate preparation
[0033] Add ethyl benzoate compound (2) (2.31g, 9.13mmol) into 20ml of dichloromethane, stir at 25°C, slowly add thionyl chloride (1.36g, 11.4mmol), and drop it at 40°C Heated to reflux for 1 hour, cooled to 0-5°C, added triethylamine (1.15g, 11.4mmol), then slowly added a solution of compound 3 (2.25g, 9.13mmol) in dichloromethane (10ml), dropwise After completion, the temperature was raised to 25°C, and the stirring reaction was continued for 3 hours, then 10×2ml of water was added to wash, dried over anhydrous sodium sulfate, the solvent was evaporated under reduced pressure, and 3.82g of the product was obtained by recrystallization in toluene-n-hexane, with a yield of 87%. .
Embodiment 2
[0035] Preparation of repaglinide
[0036] Add ethyl benzoate compound 4 (2.15g, 4.47mmol) into methanol 25ml, stir and reflux at 65°C, then slowly add 0.9mol / L sodium hydroxide solution (9.94ml, 8.94mmol) and react for 3 hours, Cool to 40-45°C, then add 0.6mol / L hydrochloric acid solution to pH ≈ 5, cool and stir in an ice bath for 0.5h, filter, wash with water, and dry 1.88g of the product, with a yield of 93%.
Embodiment 3
[0038] Methyl S(+)-2-ethoxy-4-[N-{1-(2-piperidinylphenyl)-3-methyl-1-butyl}aminocarbonylmethyl]benzoate preparation
[0039] Add methyl benzoate compound (2) (1.42g, 5.95mmol) into chloroform (12ml), stir, cool to -10°C, slowly add oxalyl chloride (0.38g, 2.98mmol), continue to React at this temperature for 3 hours, then add potassium carbonate (0.81g, 5.95mmol), heat up to 60°C, slowly add 8ml of a chloroform solution of compound 3 (1.47g, 5.95mmol), and continue to stir after the dropwise addition. hours, then add 10×2ml of water to wash, dry over anhydrous sodium sulfate, evaporate the solvent under reduced pressure, and recrystallize in toluene-n-hexane to obtain 1.49 g of the product, with a yield of 53.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com