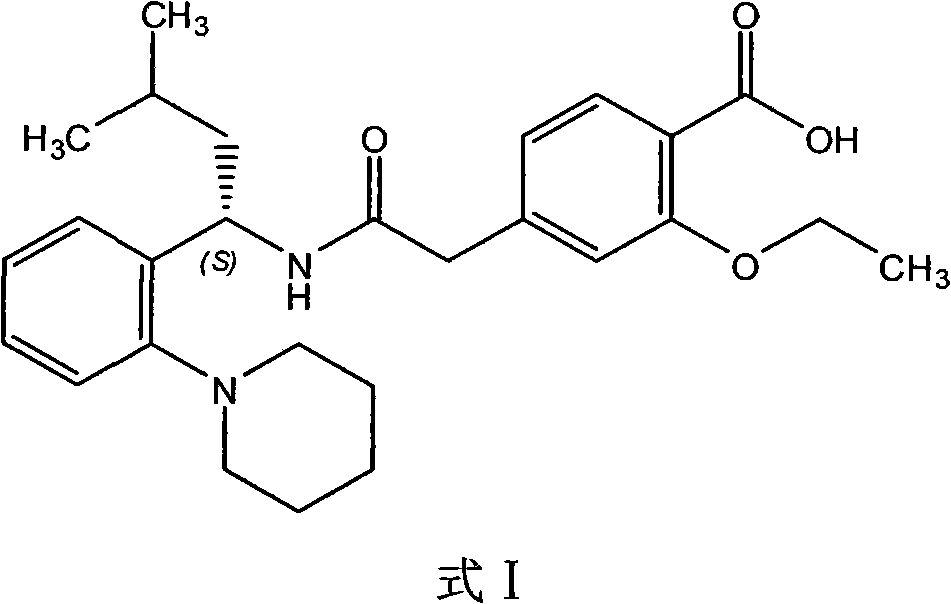
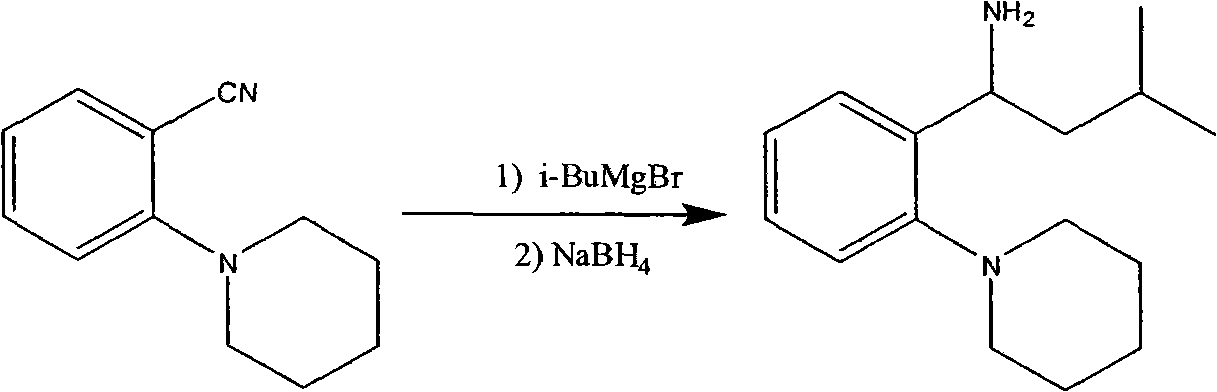One-pot method for synthesizing repaglinide for treating diabetes
A technology for oily substance and solvent, applied in the field of synthesizing repaglinide by method, can solve the problems of long production cycle, unstable production, expensive acetonitrile, etc., and achieve the effects of shortening the reaction route, simplifying the operation, and reducing pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
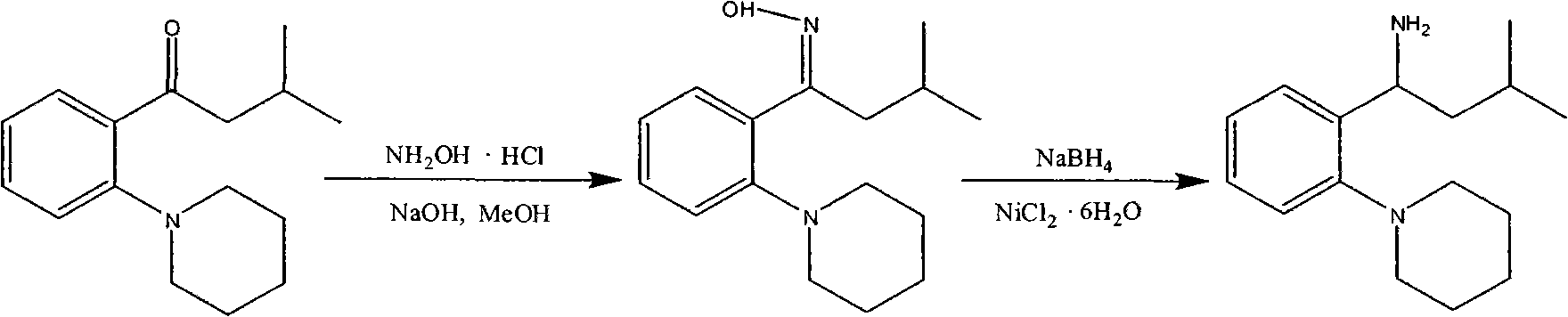
[0027] A: Preparation of (S)-3-methyl-1-[2-(1-piperidinyl)phenyl]butylamine
[0028] (S)-3-Methyl-1-(2-piperidinylphenyl)butylamine L-N-acetyl glutamate 16g, dichloromethane 75ml, tap water 20ml, stirring and cooling down to 0-5°C; add 1N hydrogen dropwise Sodium oxide solution, adjust the pH to 9-10, and dissolve all the solids. Stir at 0-5°C for 30 minutes, let stand to separate the organic phase. Add 20ml of dichloromethane to the aqueous phase and extract once more. The water phase was discarded, the combined organic phase was washed once with 100 ml of water, the water phase was discarded, and 5-10 g of anhydrous sodium sulfate was added to the organic phase, stirred and dehydrated at room temperature for 1 hour, and suction filtered. Dichloromethane was evaporated from the oil layer to dryness under reduced pressure; 30 ml of toluene was added to dissolve it, and it was directly used in the next reaction.
Embodiment 2
[0030] A: Preparation of (S)-3-methyl-1-[2-(1-piperidinyl)phenyl]butylamine
[0031] The extraction solvent is replaced with ethyl acetate, toluene, cyclohexane, etc. and the rest are the same.
Embodiment 3
[0033] B: (S)-4-(2-((4-methoxybenzyl)(3-methyl-1-(2-(1-piperidinyl)phenyl)butyl)amino)-2- Preparation of ethyl oxoethyl)-2-ethoxybenzoate
[0034] 9.25g of 4-ethoxycarbonyl-3-ethoxyphenylacetic acid, 50ml of toluene, and 4.4g of triethylamine were dissolved by stirring. Cool down to -10~-20°C, add a solution of 5g trimethylacetyl chloride and 10ml toluene, and keep it warm for 1 hour; add the (S)-3-methyl-1-(2-piperidinylbenzene base) butylamine and toluene solution. The reaction was stirred for 10 hours. The end point of the reaction was monitored by TLC. After completion of the reaction, 60ml of water was added, stirred, allowed to stand, and the water phase was discarded. The organic phase was washed once with 50 ml of salt water, the water layer was discarded, and the organic phase was concentrated to dryness with toluene to obtain an intermediate oil.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com