Method for preparing Repaglinide
A compound and a selected technology, applied in the field of preparing repaglinide, can solve the problems of increasing the difficulty and danger of industrialized production of intermediates, increasing the production cost and danger of repaglinide, increasing costs, and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
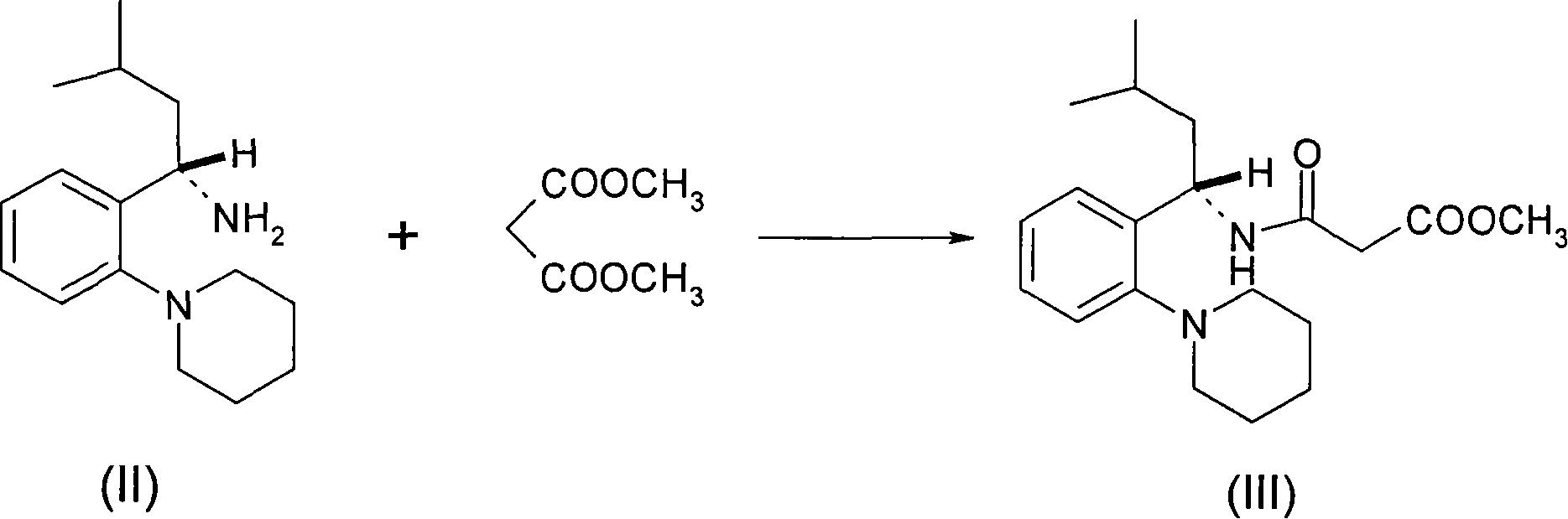
[0018] Preparation of (S)-3-(4-methyl-2-(2-(piperidin-1-yl)phenyl)pent-2-ylamino)-3-oxopropionic acid methyl ester
[0019] Dissolve (S)-3-methyl-2(2-piperidinylphenyl)-1-butylamine (12 g, 0.049 mol) in 120 ml of methanol, and add anhydrous potassium carbonate (25 g) under stirring , dimethyl malonate (20 g, 0.15 mol), reflux for 6 hours, filter while hot to remove the solid, concentrate the mother liquor to dryness, add ethyl acetate / n-hexane (1:5) to stir and crystallize, filter to obtain white Solids: 12.5 g. MP: 82-84°C.
Embodiment 2
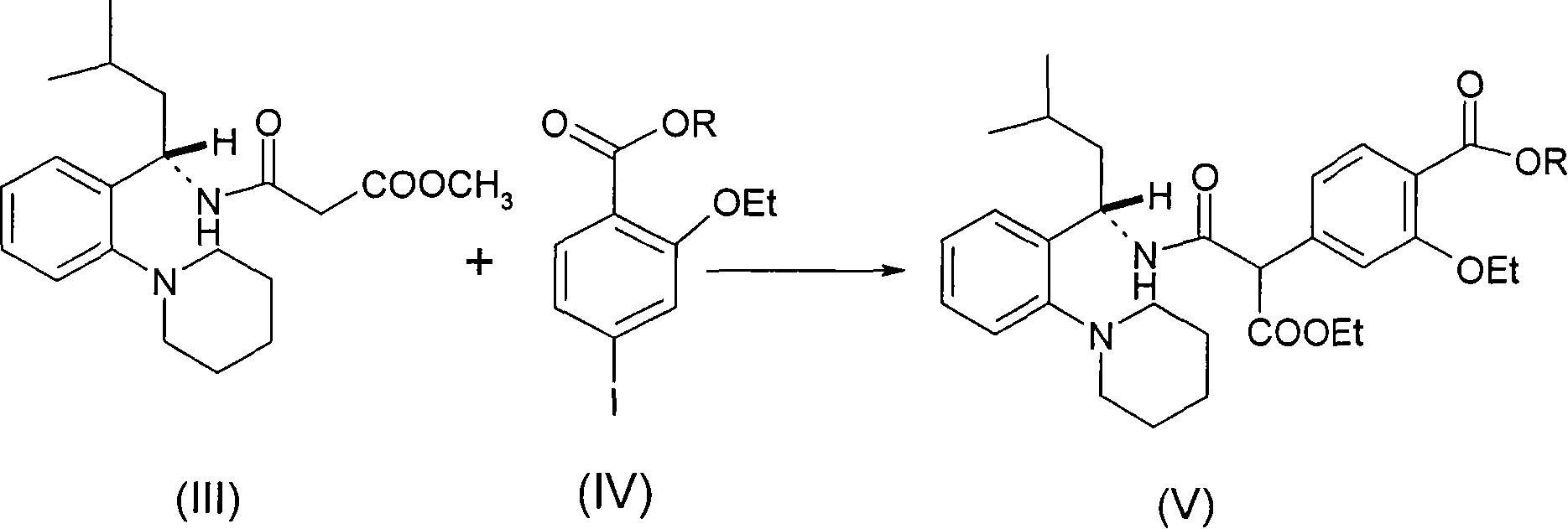
[0021] 2-ethoxy-4-(3-methoxy-1-((S)-4-methyl-2-(2-(piperidin-1-yl)phenyl-2-amino)-1, Preparation of 3-dioxopropan-2-yl) ethyl benzoate
[0022] Dissolve the intermediate (10 g, 0.029 mol, prepared in Example 1) in 100 ml of absolute ethanol, add sodium ethoxide (10 g, 0.147 mol), and cool in an ice-salt bath (-5°C-0°C) , dropwise added compound 4-iodo-2-ethoxy ethyl benzoate (8.8 g, 0.03 mol) dissolved in 50 ml of dehydrated ethanol solution, added in one hour, reacted at room temperature for 2 hours, concentrated to dryness, added 100 ml of water , extracted with ethyl acetate (50 ml×3), washed with saturated brine, dried, filtered, and concentrated to dryness to obtain a solid, which was recrystallized from ethyl acetate:n-hexane=1:8 to obtain intermediate (7): 12.3 g.
Embodiment 3
[0024] Preparation of S(+)-2-ethoxy-4-[N-{1-(2-piperidinylphenyl)-3-methyl-1-butyl}aminocarbonylmethyl]benzoic acid
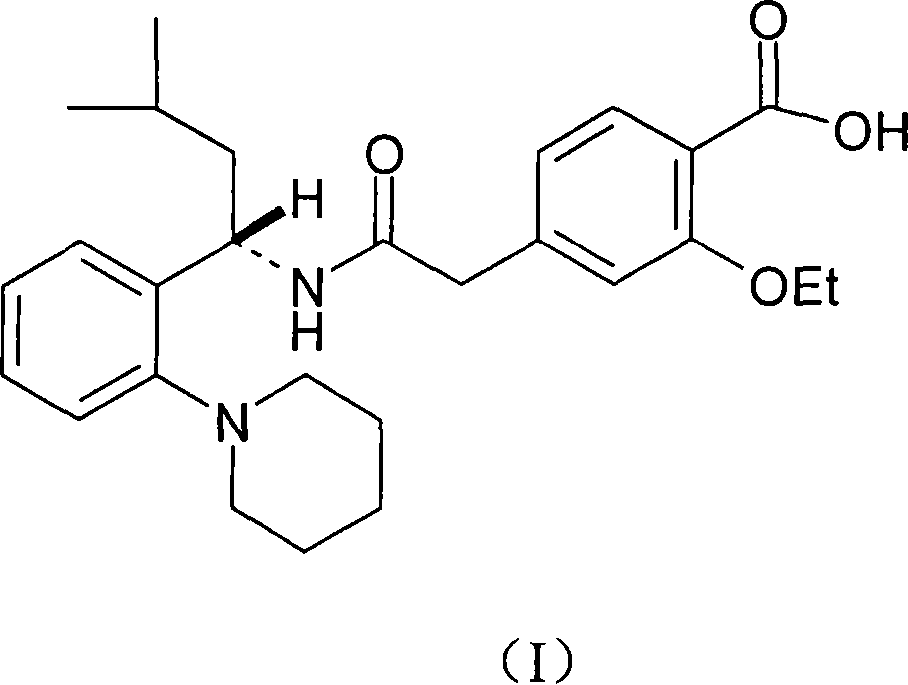
[0025] Dissolve the intermediate (12 grams, 0.0235 moles, prepared in Example 2) in 120 milliliters of methanol, add 40 milliliters of water, 6 grams of sodium hydroxide (0.15 moles) and stir under reflux for 3 hours, concentrate to remove most of the solvent, add Concentrated hydrochloric acid 13.5 ml, slowly heated to above 80°C until bubbles appeared, then heated to 100°C, kept warm for 30 minutes, added 120 ml of water, stirred, adjusted to PH=4, cooled, filtered, and washed with water to obtain the target compound, Rui Glinide: 9.6 grams.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com