Repaglinide tablet and preparation method thereof
A repaglinide tablet, weight percentage technology, applied in the field of pharmaceutical preparations, can solve the problems of difficulty in improving product quality, inability to improve dissolution, poor adhesion and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
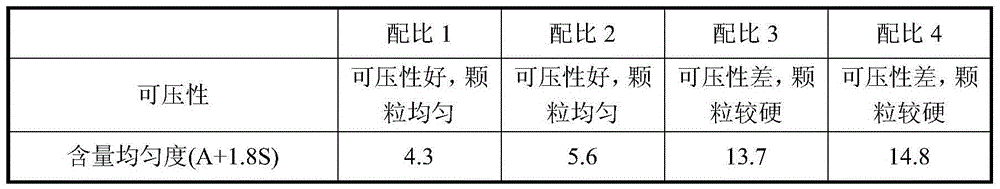
[0074] The weight percentage of each component of the tablet consists of: repaglinide 1%, microcrystalline cellulose 49%, calcium hydrogen phosphate 25%, pregelatinized starch 10%, croscarmellose sodium 5.5%, polyvinyl chloride Ketone 5%, Poloxamer 188 (F68) 1.5%, Meglumine 2%, Magnesium Stearate 0.5%, Yellow Iron Oxide 0.25%, Red Iron Oxide 0.25%.
[0075] Preparation method, the steps are as follows:
[0076] 1) Pass the raw material and auxiliary materials microcrystalline cellulose, calcium hydrogen phosphate, pregelatinized starch, croscarmellose sodium, magnesium stearate, coloring agent, etc. through a 60-mesh sieve, and set aside.
[0077] 2) Mix the coloring agent with croscarmellose sodium, repaglinide raw material and pregelatinized starch; after mixing, add calcium hydrogen phosphate and 1 / 3 of microcrystalline cellulose and mix in a three-dimensional motion mixer After 5 to 10 minutes, put the rest of the microcrystalline cellulose in the prescribed amount into a...
Embodiment 2
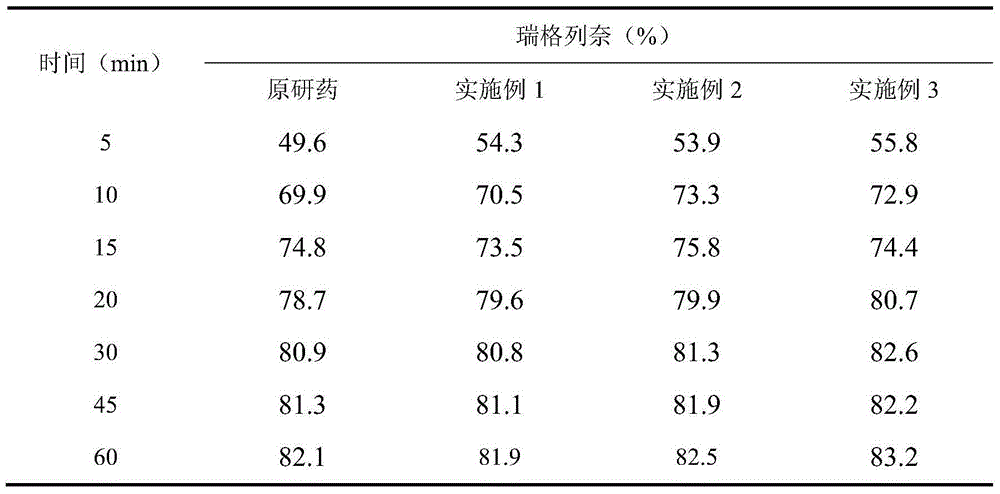
[0082] Repaglinide 1%, microcrystalline cellulose 51.5%, calcium hydrogen phosphate 25%, pregelatinized starch 10%, croscarmellose sodium 5%, povidone 3%, poloxamer 188 ( F68) 1.5%, meglumine 2%, magnesium stearate 0.5%, yellow iron oxide 0.25%, red iron oxide 0.25%.
[0083] Preparation method, the steps are as follows:
[0084] 1) Pass the raw material and auxiliary materials microcrystalline cellulose, calcium hydrogen phosphate, pregelatinized starch, croscarmellose sodium, magnesium stearate, coloring agent, etc. through a 60-mesh sieve, and set aside.
[0085] 2) Mix the coloring agent with croscarmellose sodium, repaglinide raw material and pregelatinized starch; after mixing, add calcium hydrogen phosphate and 1 / 3 of microcrystalline cellulose and mix in a three-dimensional motion mixer After 5 to 10 minutes, put the rest of the microcrystalline cellulose in the prescribed amount into a three-dimensional motion mixer and mix for 30 minutes.
[0086] 3) Take the presc...
Embodiment 3
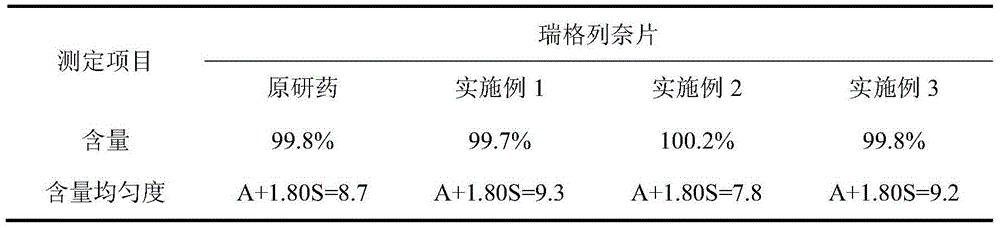
[0090] Repaglinide 2%, microcrystalline cellulose 50.5%, calcium hydrogen phosphate 25%, pregelatinized starch 10%, croscarmellose sodium 5%, povidone 3%, poloxamer 188 ( F68) 1.5%, meglumine 2%, magnesium stearate 0.5%, yellow iron oxide 0.25%, red iron oxide 0.25%.
[0091] Preparation method, the steps are as follows:
[0092] 1) Pass the raw material and auxiliary materials microcrystalline cellulose, calcium hydrogen phosphate, pregelatinized starch, croscarmellose sodium, magnesium stearate, coloring agent, etc. through a 60-mesh sieve, and set aside.
[0093] 2) Mix the coloring agent with croscarmellose sodium, repaglinide raw material and pregelatinized starch; after mixing, add calcium hydrogen phosphate and 1 / 3 of microcrystalline cellulose and mix in a three-dimensional motion mixer After 5 to 10 minutes, put the rest of the microcrystalline cellulose in the prescribed amount into a three-dimensional motion mixer and mix for 30 minutes.
[0094] 3) Take the presc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com