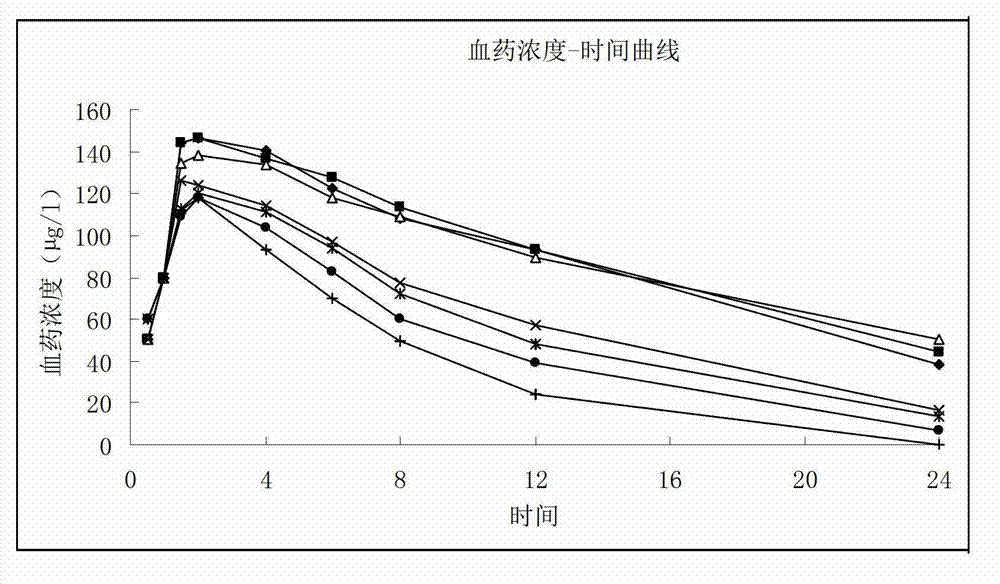
Calcium and zinc gluconate liposome oral solution
A technology of calcium zinc gluconate and oral solution, which is applied in the field of liposome oral solution and its preparation method, calcium zinc gluconate liposome oral solution, and can solve the problem of no improvement, little difference in substance, and low bioavailability and other problems, to achieve the effect of long residence time, high encapsulation rate and high bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0057] According to another aspect of the present invention, the preparation method of above-mentioned calcium zinc gluconate liposome oral solution is provided, the method may further comprise the steps:
[0058] (1) Stir and sonicate egg yolk lecithin, soybean sterol, phosphatidylglycerol and cholesterol in water for injection for 20 minutes, then perform gradient homogenization for 5 to 7 times under a pressure of 300bar-1000bar to obtain a lipid solution;
[0059] (2) Add calcium gluconate, zinc gluconate and lysine hydrochloride to the lipid solution prepared above, heat to 60°C, keep stirring and sonicate for 50 minutes;
[0060] (3) After dissolving trehalose in water for injection, pour the above solution into it, dilute to volume with water for injection, stir evenly, filter through a 0.45 μm microporous membrane, and fill to obtain calcium zinc gluconate liposome oral solution.
[0061] In the method according to the present invention, the gradient homogenization is ...
Embodiment 1
[0066] The preparation of embodiment 1 calcium zinc gluconate liposome oral solution
[0067] The raw materials used are as follows:
[0068]
[0069] The preparation process is as follows:
[0070] (1) Mix 120g of egg yolk lecithin, 20g of phosphatidylglycerol, 20g of soybean sterol and 20g of cholesterol in 800ml of water for injection and ultrasonically stir for 20min, then homogenize twice under 300bar pressure, twice under 800bar pressure, and finally 900bar Homogenize twice again to obtain a lipid solution;
[0071] (2) Add 60g of calcium gluconate, 3g of zinc gluconate and 10g of lysine hydrochloride to the lipid solution prepared above, heat to 60°C, keep stirring and ultrasonicate for 50min;
[0072] (3) Dissolve 55g of trehalose in an appropriate amount of water for injection, pour the above solution, add water for injection to 1000ml, stir evenly, filter with a 0.45μm microporous membrane, fill 10ml / bottle, and obtain 100 bottles of calcium zinc gluconate Lipo...
Embodiment 2
[0073] The preparation of embodiment 2 calcium zinc gluconate liposome oral solution
[0074] The raw materials used are as follows:
[0075]
[0076]
[0077] The preparation process is as follows:
[0078] (1) Mix 150g of egg yolk lecithin, 30g of phosphatidylglycerol, 30g of soybean sterol and 20g of cholesterol in 800ml of water for injection and ultrasonically stir for 20min, then homogenize once under 500bar pressure, 3 times under 500bar pressure, and finally 1000bar Homogenize twice again to obtain a lipid solution;
[0079] (2) Add 60g of calcium gluconate, 3g of zinc gluconate and 10g of lysine hydrochloride to the lipid solution prepared above, heat to 60°C, keep stirring and ultrasonicate for 50min;
[0080] (3) Dissolve 70g of trehalose in an appropriate amount of water for injection, pour the above solution, add water for injection to 1000ml, stir evenly, filter with a 0.45μm microporous membrane, fill 10ml / bottle, and obtain 100 bottles of calcium zinc g...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com