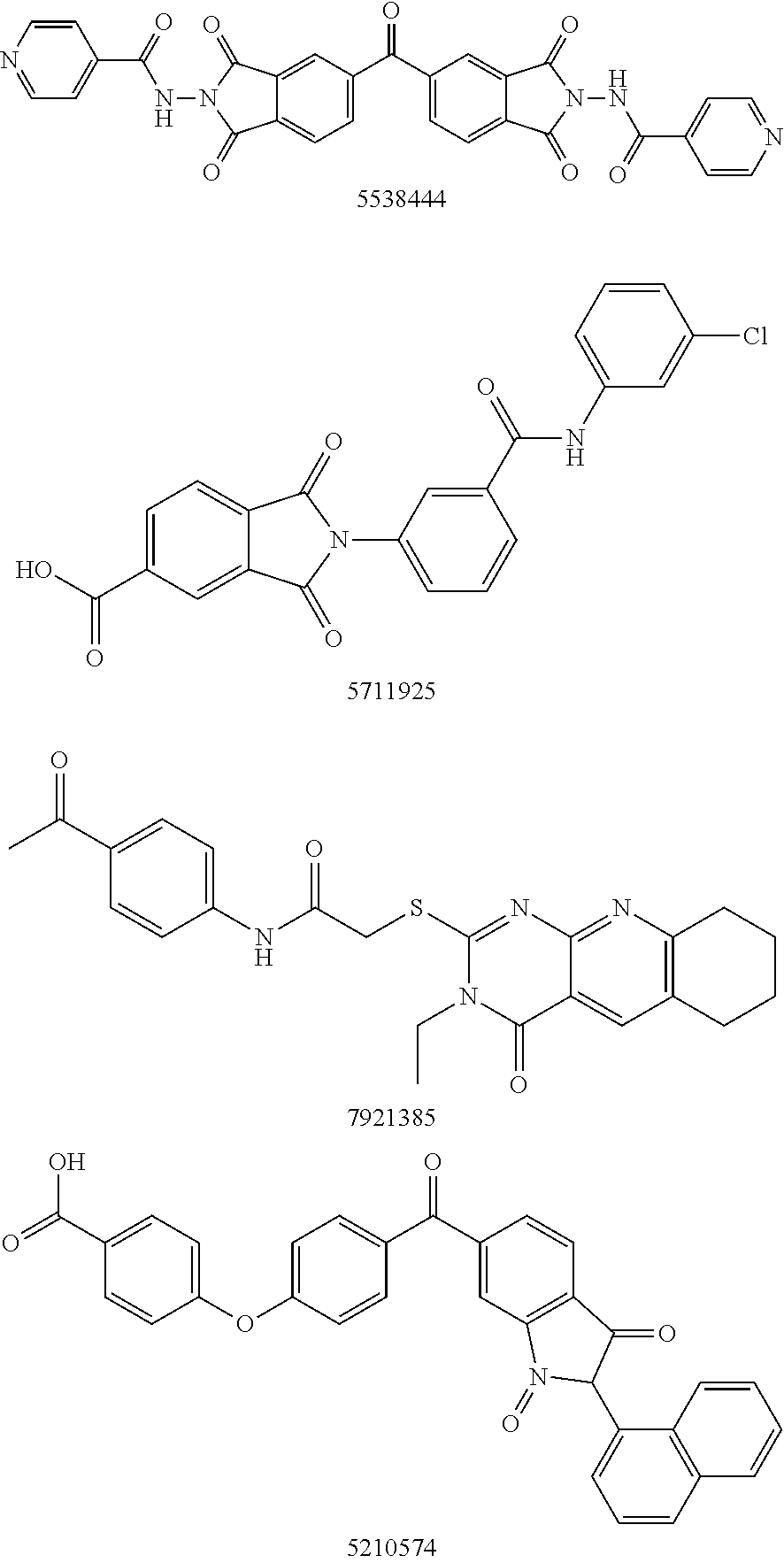
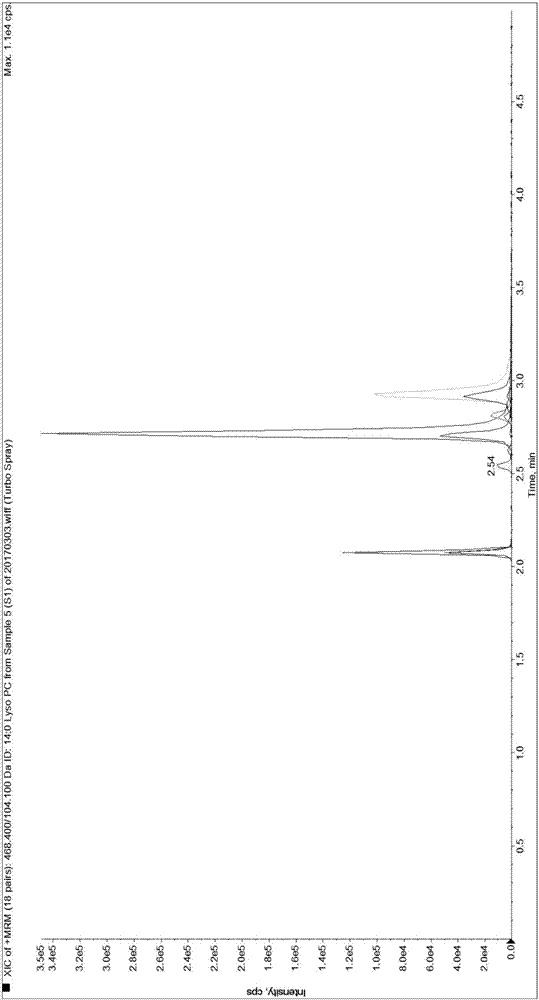
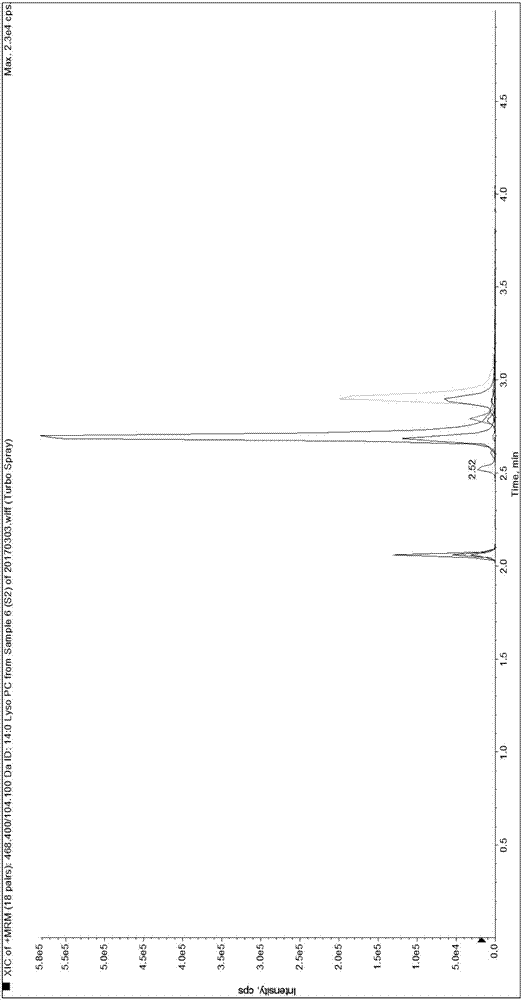
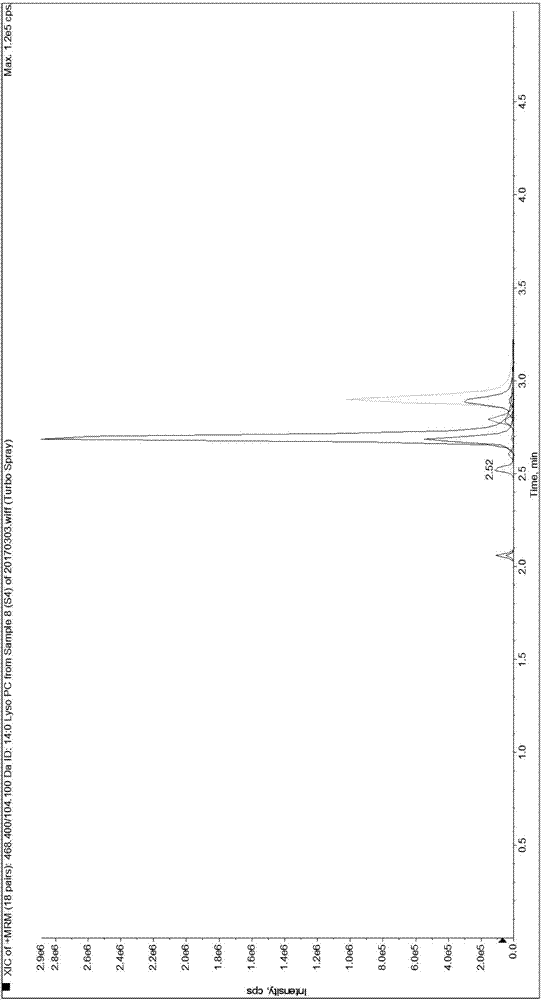
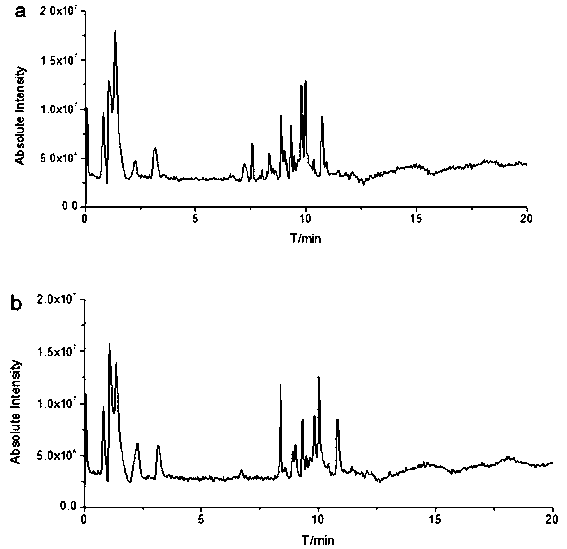
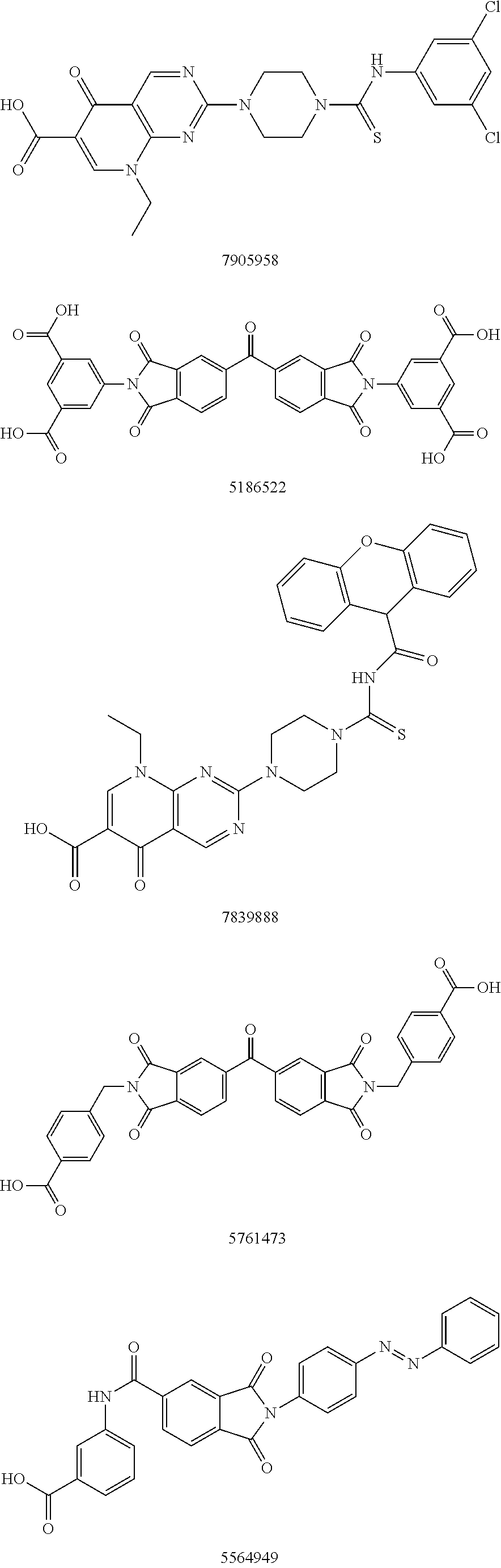
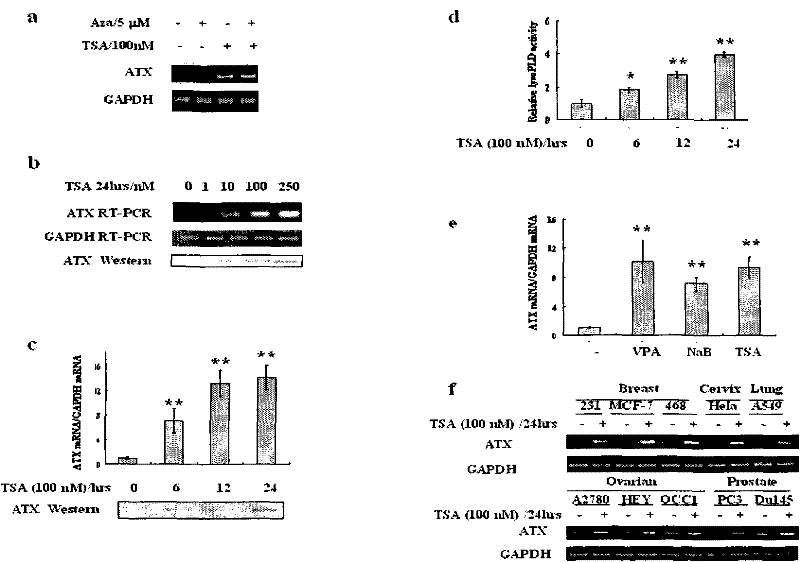
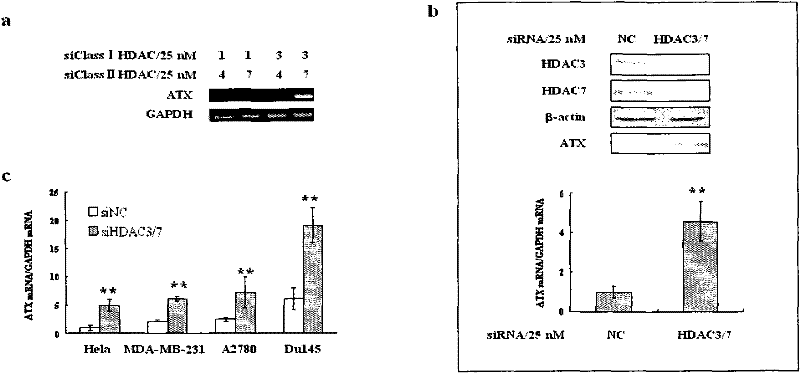
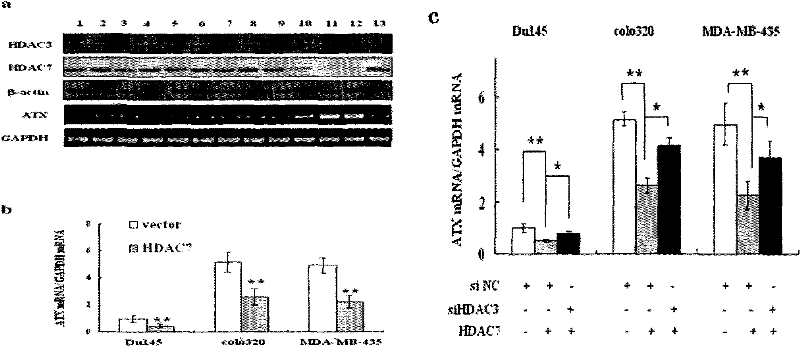
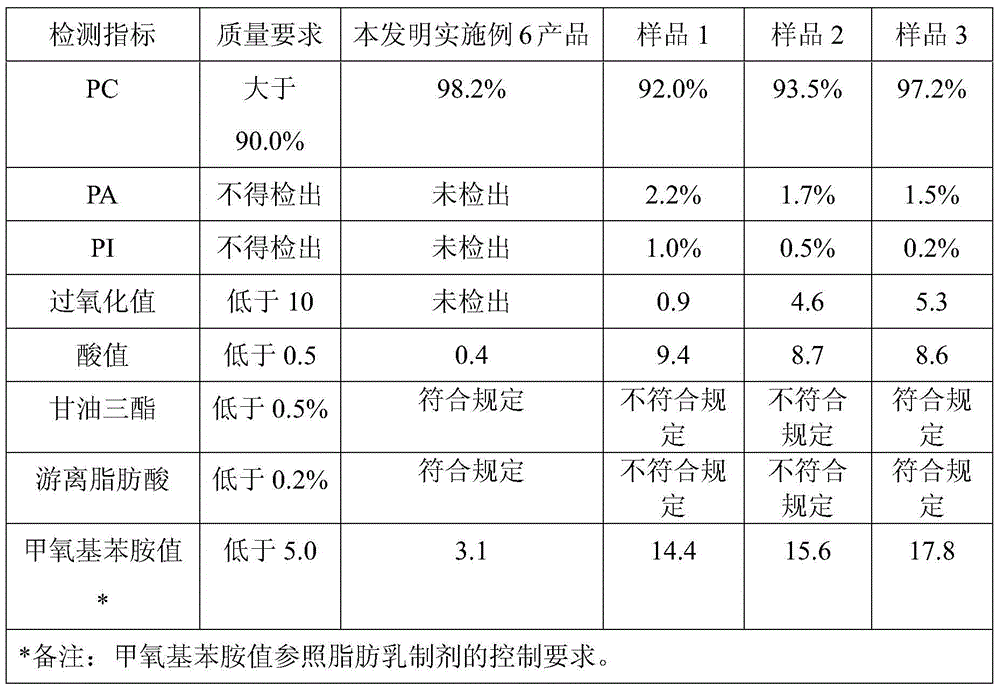
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
31 results about "Lysophosphatidyl choline" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
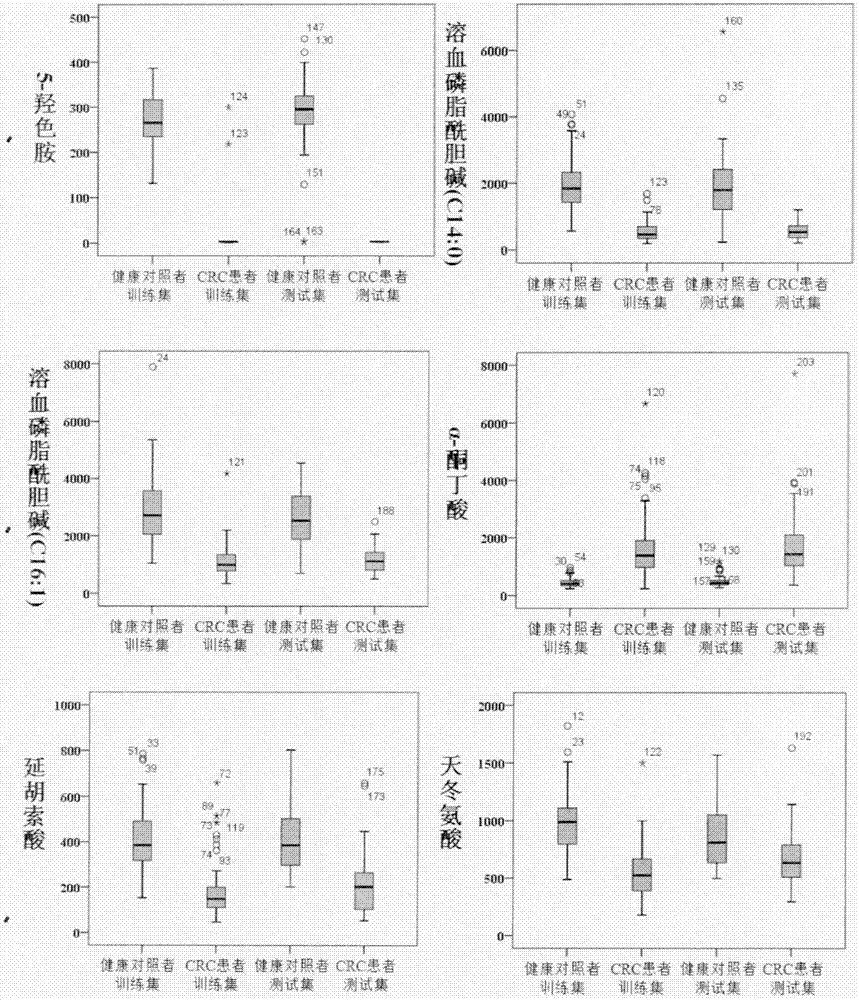
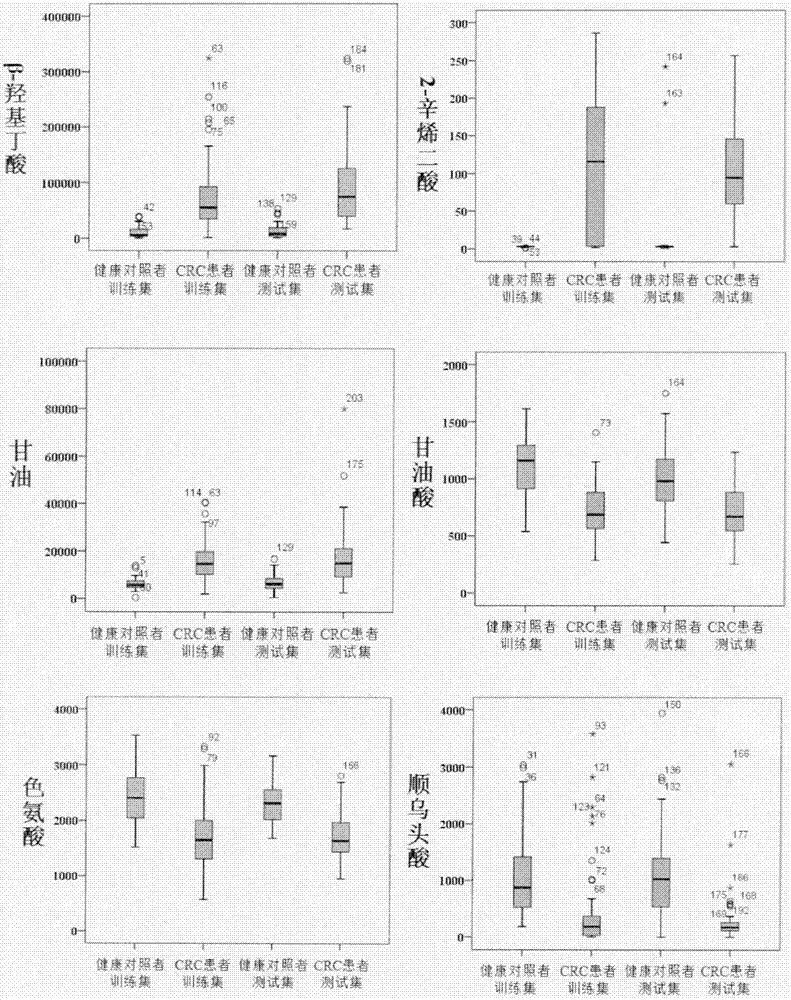
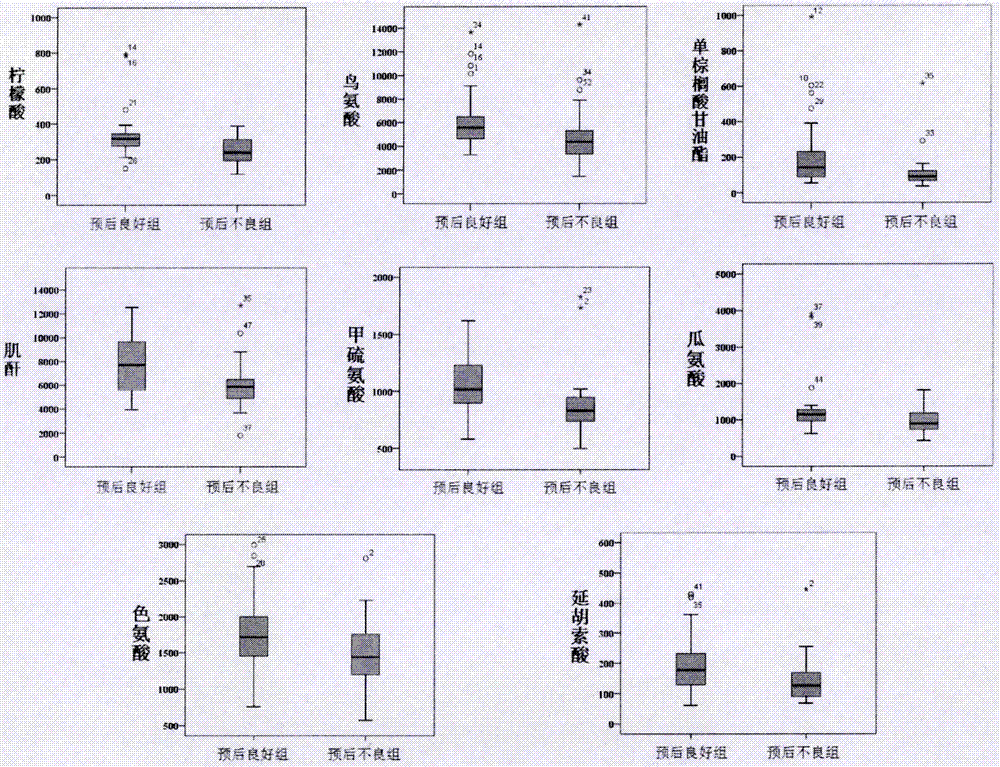
Molecular marker with auxiliary functions of early diagnosis and prognosis monitoring on colorectal cancer, and application thereof
Owner:SHENZHEN UNIV
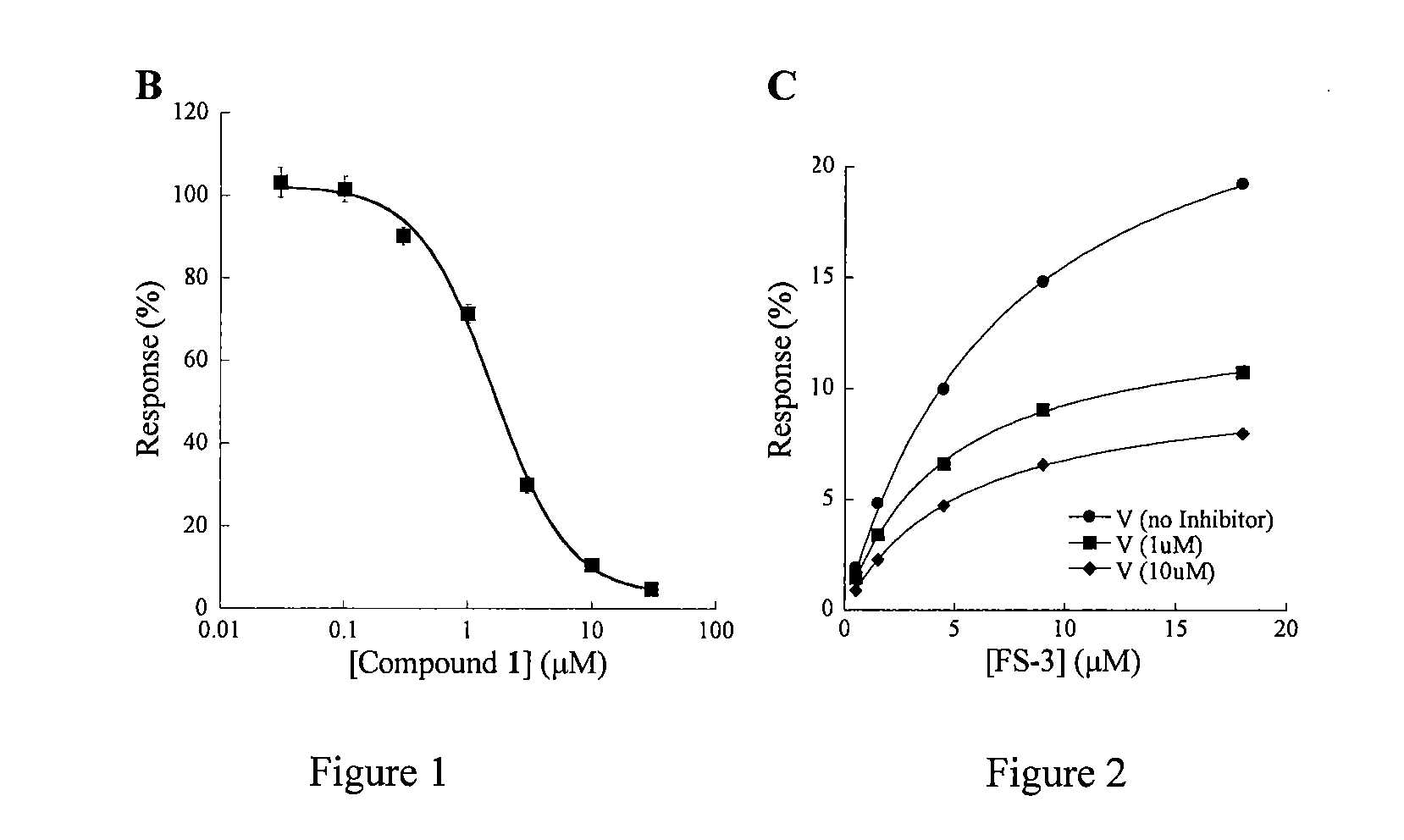
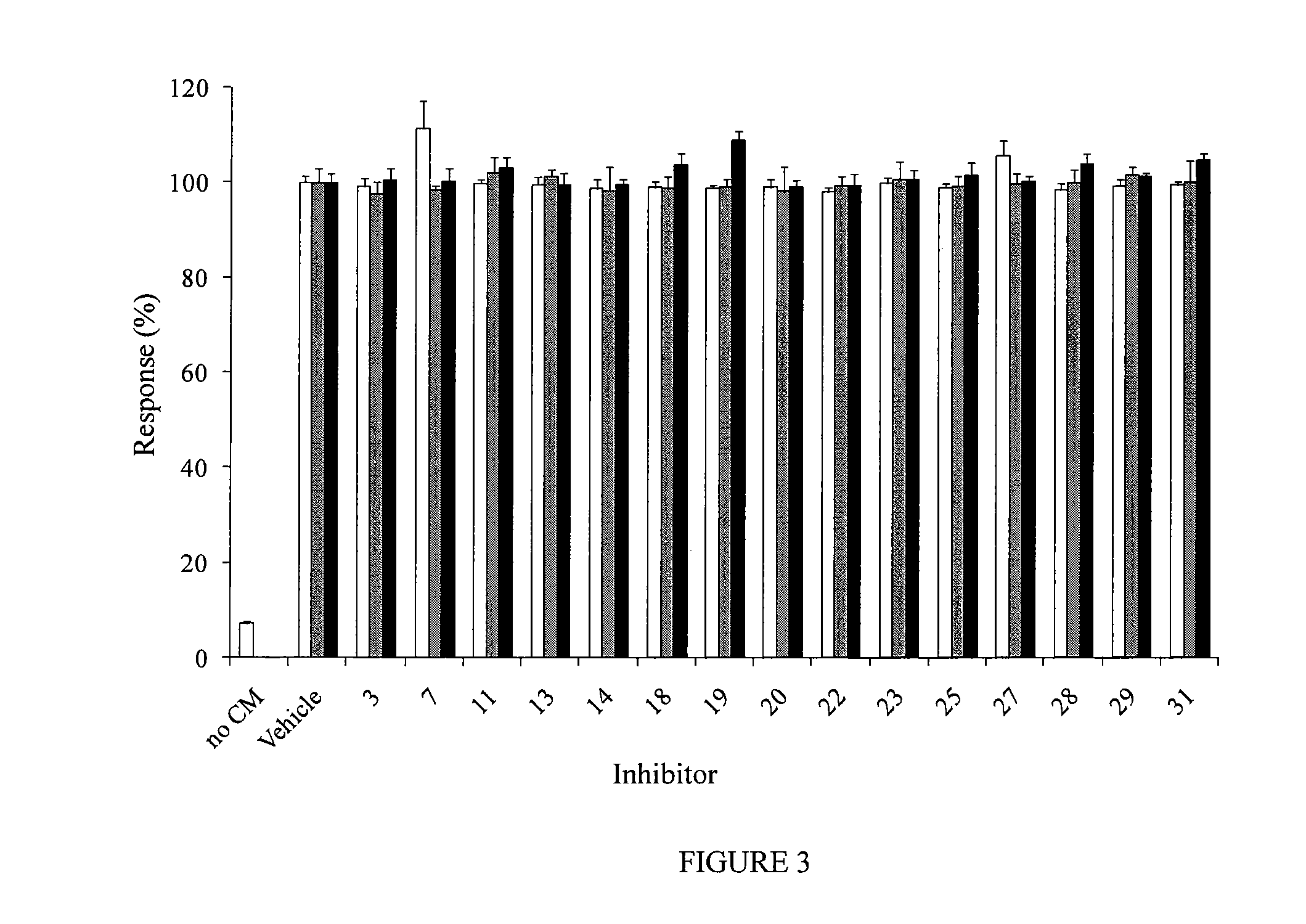
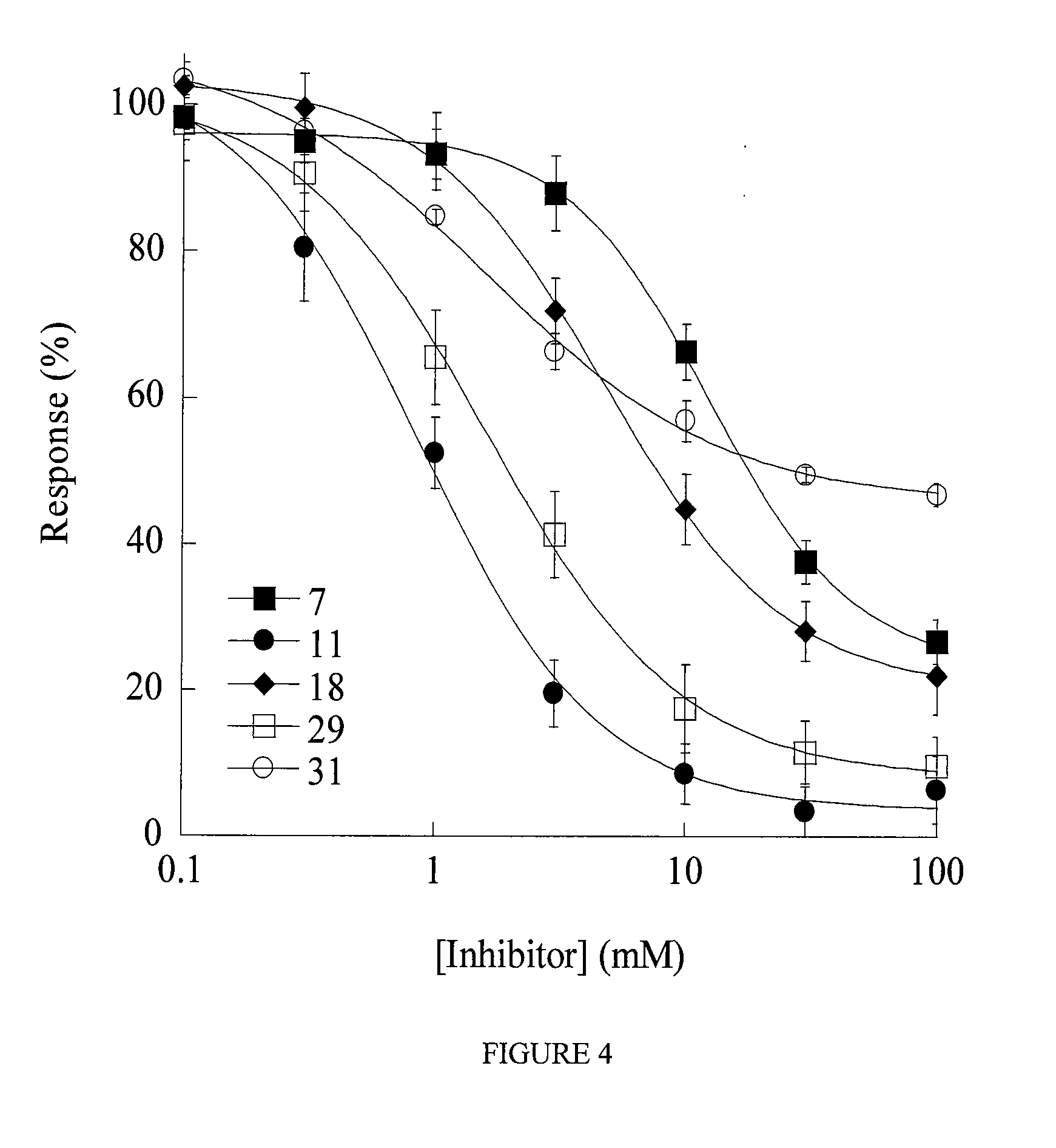
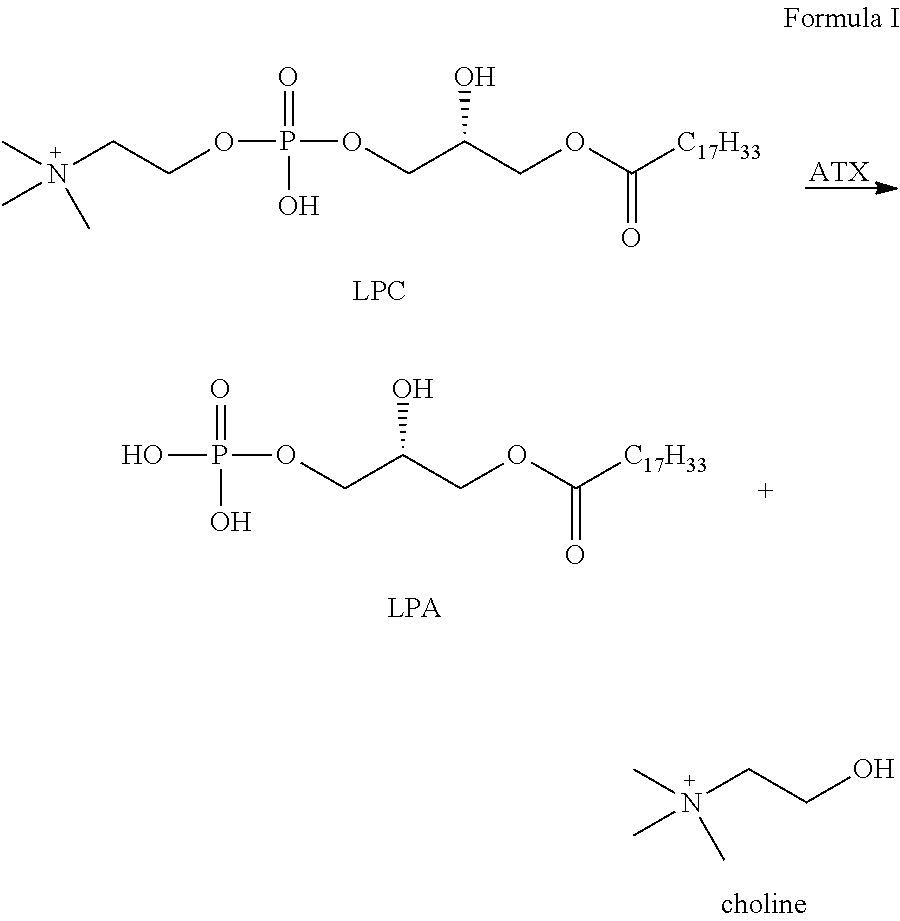
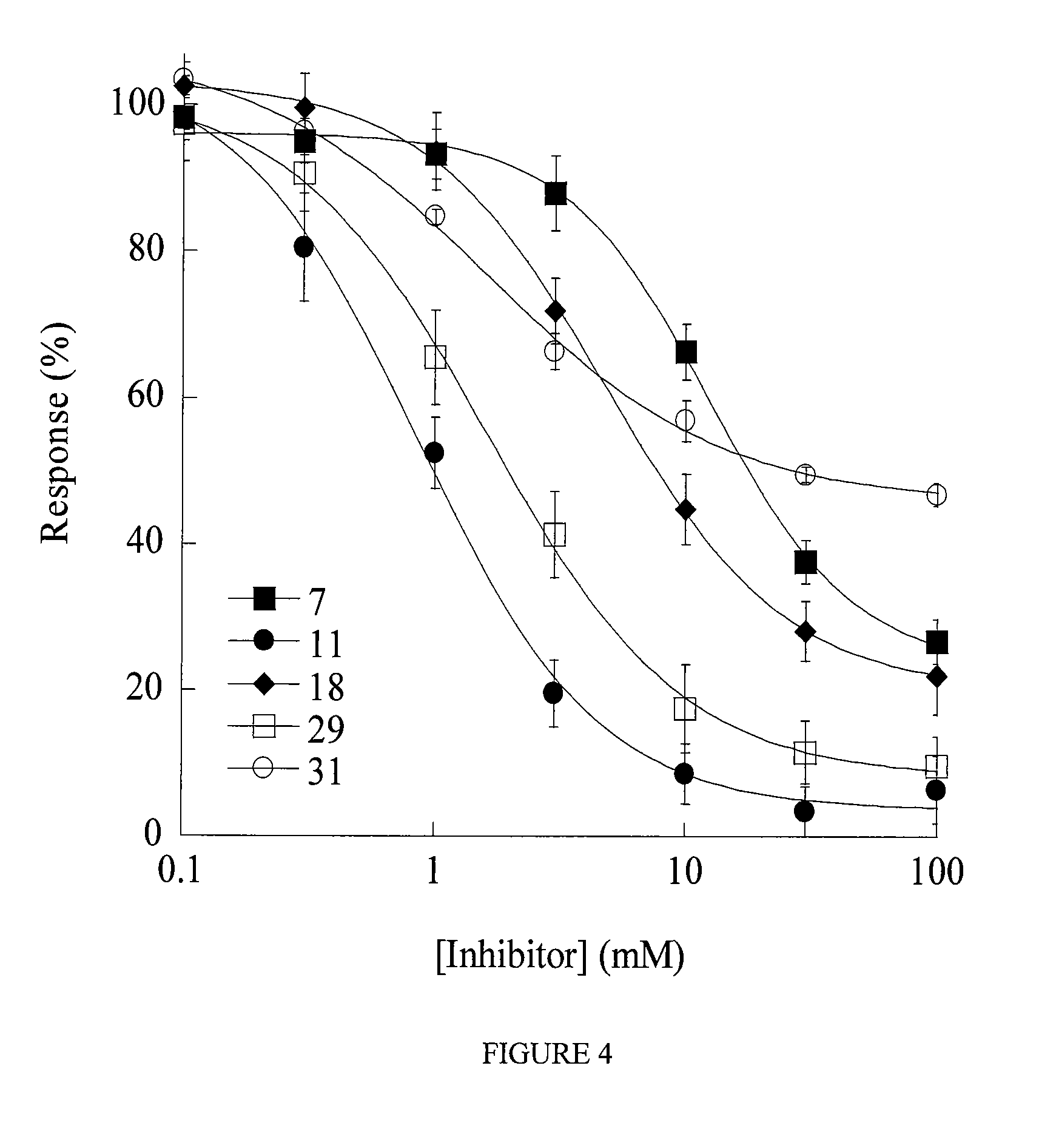
Pipemidic acid derivative autotaxin inhibitors
InactiveUS20120100592A1Reliable and highly effectiveReduce conversionPeptidesChemical treatment enzyme inactivationAutotaxinHuman body
Novel and optimized classes of pipemidic acid derivative compounds that exhibit effective inhibition of autotaxin enzymes are provided. Such classes of compounds exhibit exhibit reactivity with autotaxin to ultimately reduce the size of the reactive sites thereon to prevent conversion of lysophosphatidyl choline to lysophophatidic acid. Furthermore, such compounds can be incorporated within delivery forms for human ingestion. As such, these compounds accord an excellent manner of potentially reducing generation of certain cancers attributable to the presence of naturally occurring autotaxin within the human body. Methods of inactivating autotaxin to certain degrees therewith such compounds are encompassed within invention as well.
Owner:UNIVERSITY OF MEMPHIS RESEARCH FOUNDATION
Autotaxin inhibitors
Classes of compounds that exhibit effective inhibition of autotaxin enzymes are provided. Such classes include thioureas, diphenyldiazerenes, xanthenes, and isoindoles and exhibit reactivity with autotaxin to ultimately reduce the size of the reactive sites thereon to prevent conversion of lysophosphatidyl choline to lysophophatidic acid. Furthermore, such compounds can be incorporated within delivery forms for human ingestion. As such, these compounds accord an excellent manner of potentially reducing generation of certain cancers attributable to the presence of naturally occurring autotaxin within the human body. Methods of inactivating autotaxin to certain degrees therewith such compounds are encompassed within invention as well.
Owner:UNIVERSITY OF MEMPHIS RESEARCH FOUNDATION
Mechanism-based inactivators of autotaxin
InactiveUS8022239B2Reduce conversionReadily availableChemical treatment enzyme inactivationPhosphorus organic compoundsReactive siteCytotoxicity
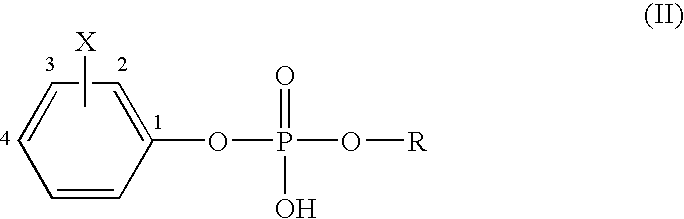
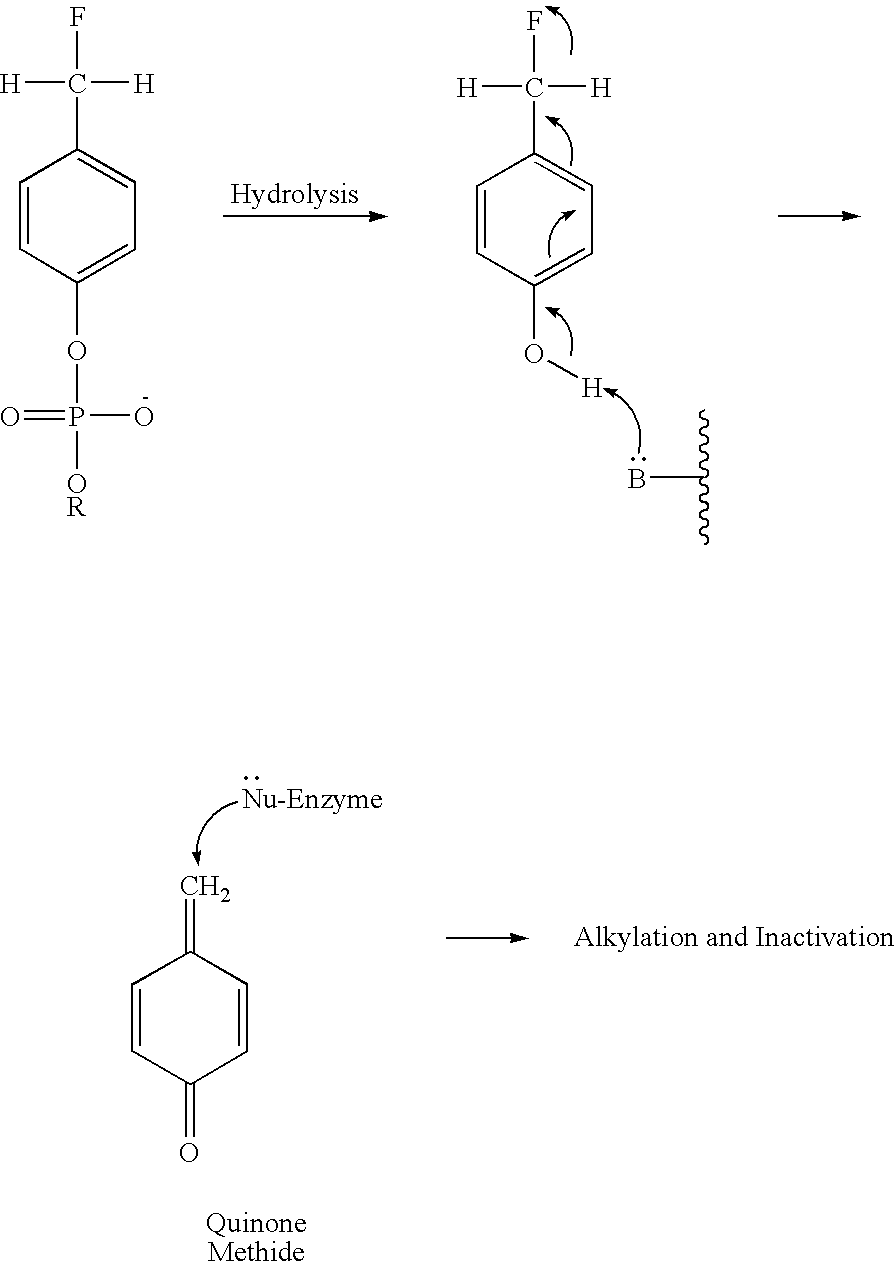
A novel class of compounds to inactivate autotaxin enzymes is provided. Such compounds include mono- and di-fluoromethylphenyl C12-C18 phosphodiesters and exhibit reactivity with autotaxin to ultimately reduce the size of the reactive sites thereon to prevent conversion of lysophosphatidyl choline to lysophophatidic acid. Furthermore, such compounds are non-cytotoxic, and can be incorporated within delivery forms for human ingestion. As such, these compounds accord an excellent manner of potentially reducing generation of certain cancers attributable to the presence of naturally occurring autotaxin within the human body. Methods of producing such novel compounds are encompassed within this invention as well as methods of inactivating autotaxin to certain degrees therewith.
Owner:UNIVERSITY OF MEMPHIS RESEARCH FOUNDATION
Lung cancer diagnosis marker combination and application
ActiveCN109946390AHigh sensitivityImprove featuresComponent separationLysophosphatidyl ethanolamineMetabolite
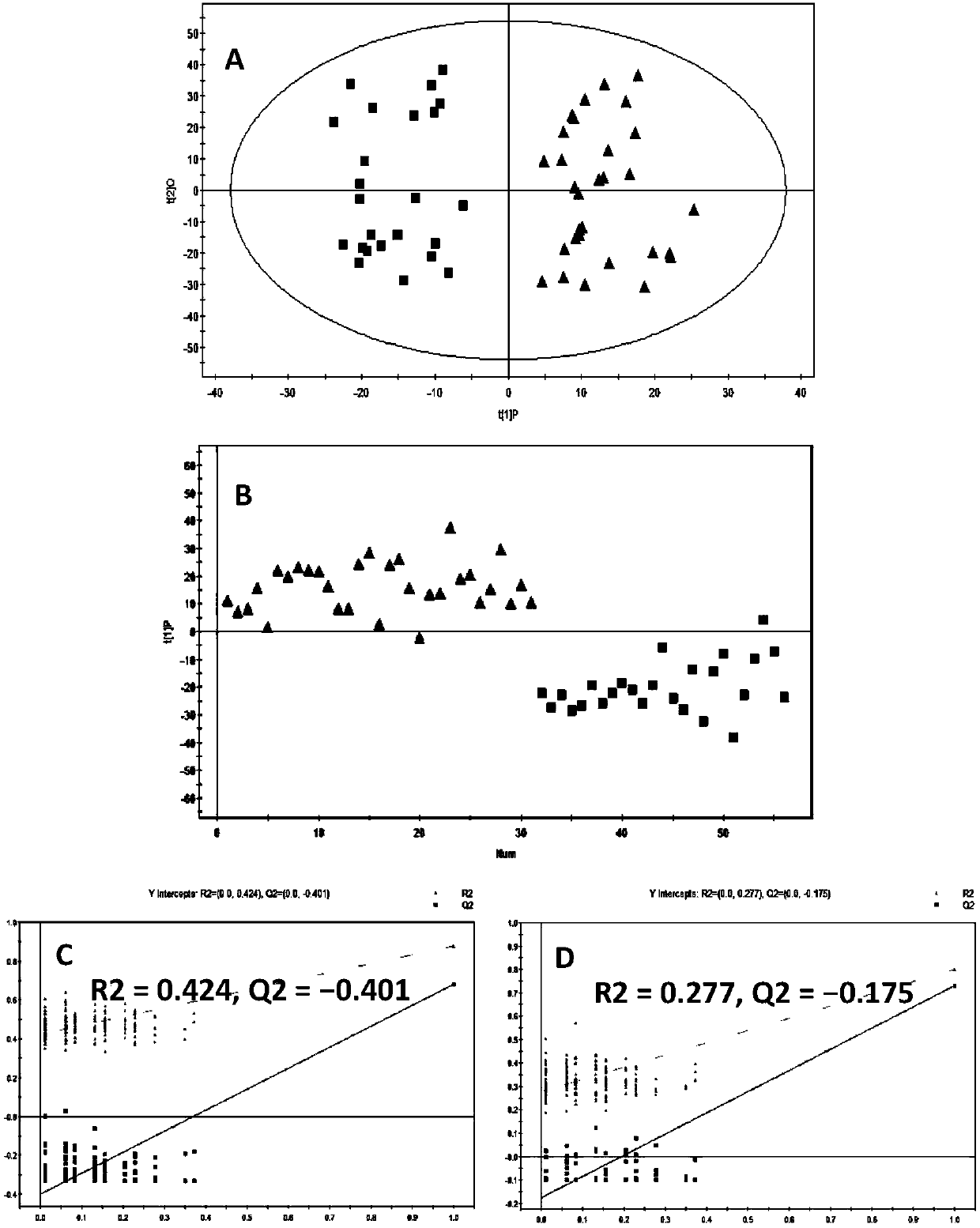
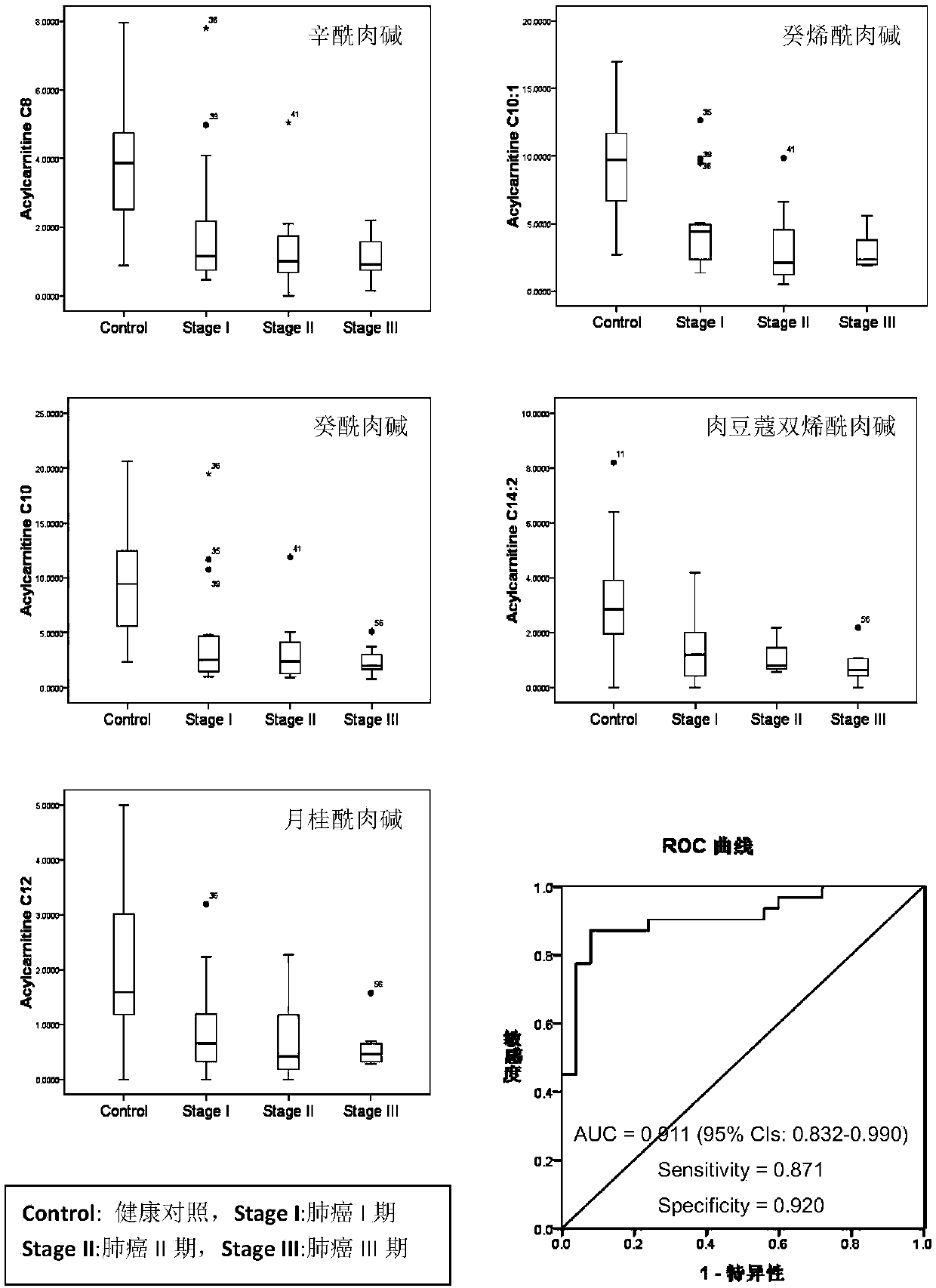
The invention discloses a diagnosis marker combination suitable for early screening and diagnosis of lung cancer. The diagnosis marker combination comprises one of 14 serum (or plasma) metabolites ormore: Acylcarnitine C8, decenoyl-carnitine (Acylcarnitine C10: 1), decanoyl-carnitine (Acylcarnitine C10), lauryl-carnitine (Acylcarnitine C12), dimyristoyl-carnitine (Acylcarnitine C14: 2); lysophosphatidyl-ethanolamine (16: 0) (LPE(16: 0)), lysophosphatidyl-ethanolamine (20: 4) (LPE (20: 4)), lysophosphatidyl-ethanolamine (20: 3) (LPE (20:3)), lysophosphatidyl-choline (20: 3 / 0: 0) (LPC (20: 3 / 0:0)), lysophosphatidyl-choline (0: 0 / 22: 5) (LPC (0: 0 / 22: 5)); 3-hydroxyl lauroleic acid (FA(12: 1(OH))), 3-hydroxy myristic dienoic acid (FA(14: 2(OH))), 3-hydroxyl linoleic acid (FA (18: (OH))), 3-hydroxy oleic acid (FA (18: 1(OH))). The diagnosis marker can be used for constructing a diagnosis model, and the model is high in sensitivity and good in specificity, can be used for early screeningand diagnosis of lung cancer, and has good clinical use and popularization values.
Owner:上海市生物医药技术研究院
Pipemidic acid derivative autotaxin inhibitors
InactiveUS8497371B2Reduce conversionReliable and highly effective autotaxin inactivatorsPeptidesChemical treatment enzyme inactivationPipemidic acidAutotaxin
Owner:UNIVERSITY OF MEMPHIS RESEARCH FOUNDATION
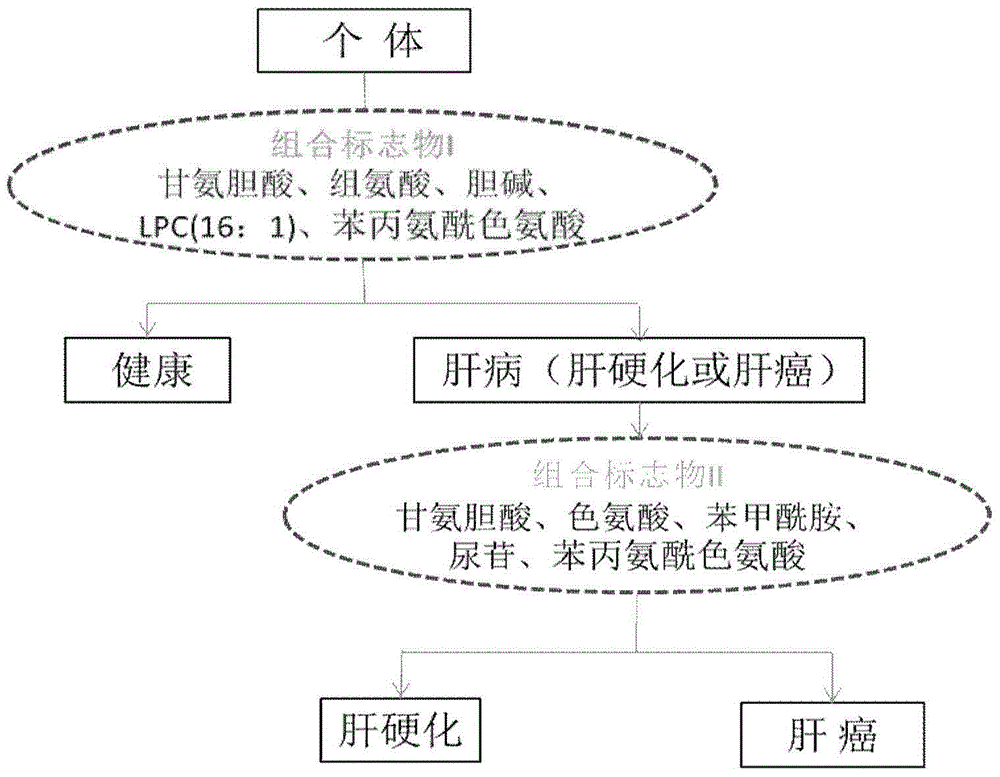
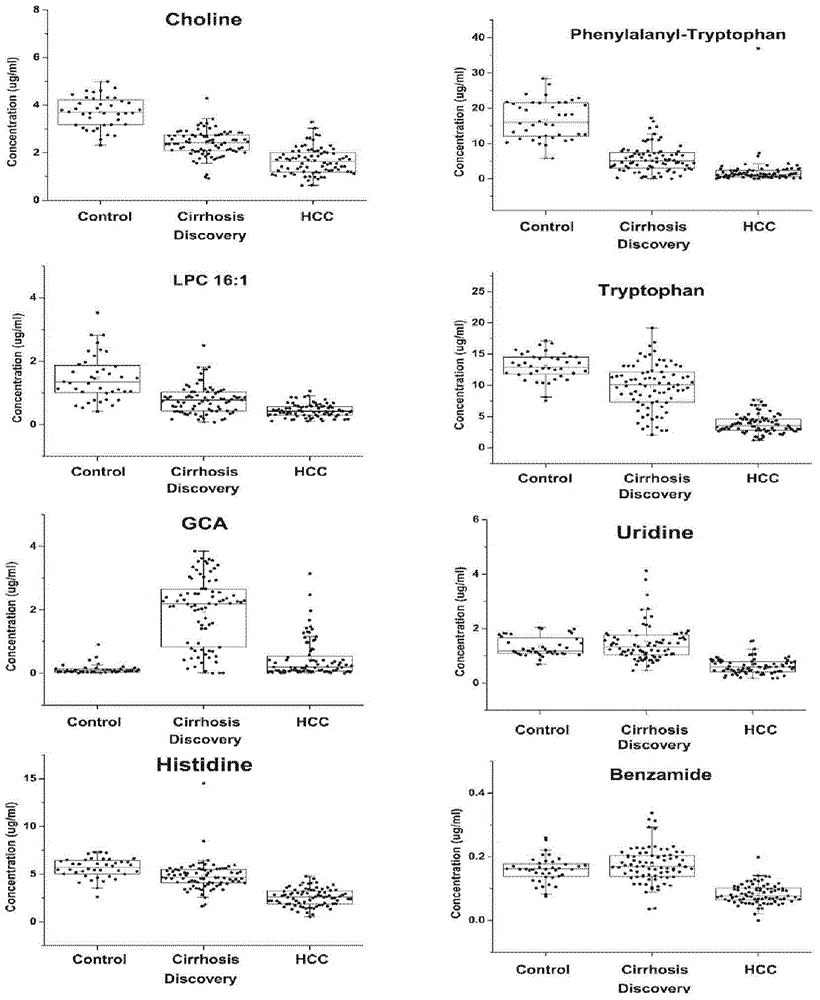
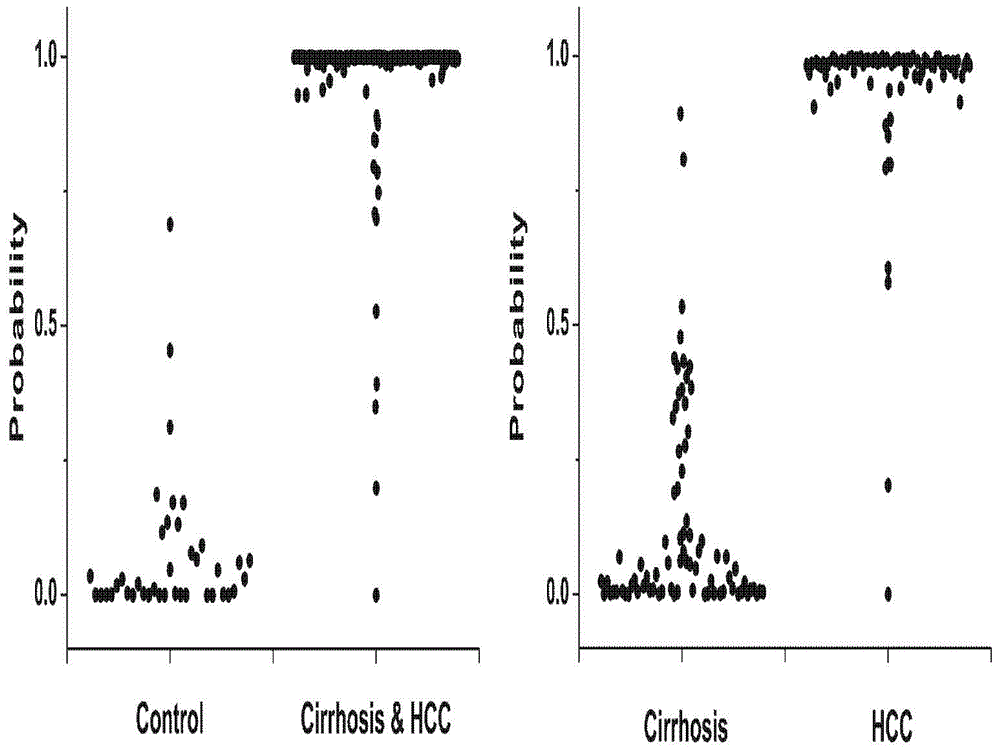
Novel serum metabolite composition and application thereof as liver cancer diagnosis marker
ActiveCN105738626AMaintain stabilityHas the value of clinical development and applicationMaterial analysisTryptophanUridine Nucleotides
The invention discloses a novel serum metabolite composition and an application thereof as a marker for preparing an early-stage liver cancer diagnosis kit. The serum metabolite composition includes: glycocholic acid, tryptophan, histidine, uridine, choline, benzamide, lysophosphatidyl choline 16:1 and phenylalanyl tryptophan. The serum metabolite composition can be used for early-stage diagnosis of liver cancer, is low in detection cost, is high in repeatability and sensitivity, and has excellent complementarity with a conventional clinical diagnosis marker serum alpha fetoprotein (AFP).
Owner:HANGZHOU HEALTH BANK MEDICAL LAB CO LTD
Pancreatic ductal adenocarcinoma marker and screening method thereof
ActiveCN109239210AImprove diagnosis rateAccurately reflect differences in metabolic profilesComponent separationPancreas Ductal AdenocarcinomaMetabolite
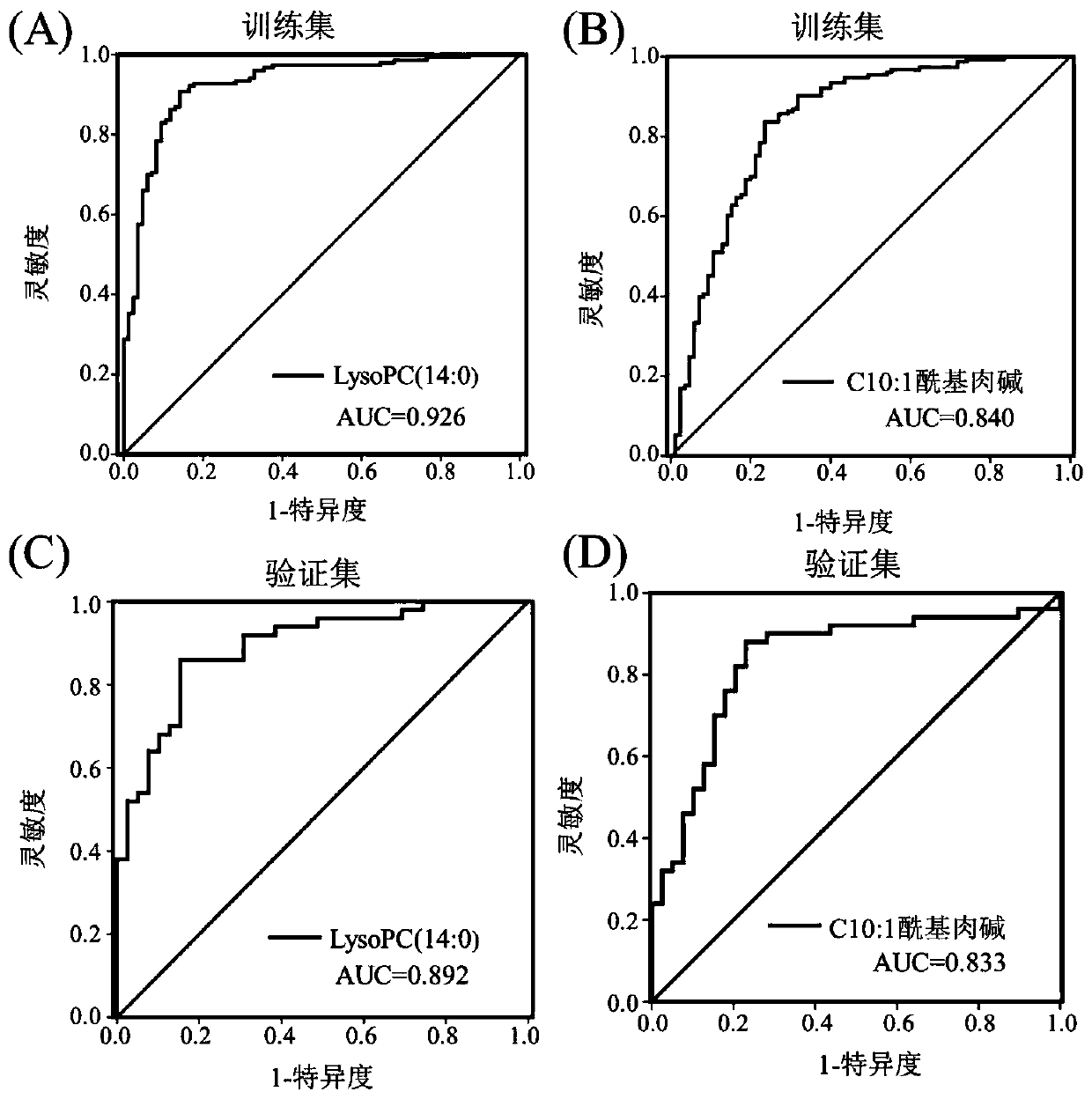
The invention discloses a pancreatic ductal adenocarcinoma marker and a screening method thereof, which belongs to the field of clinical test and diagnosis. In view of the problem that the detection sensitivity and the detection specificity of the existing pancreatic ductal adenocarcinoma diagnosis marker are poor, serum of an early patient of early stage pancreatic duct adenocarcinoma is subjected to a trace metabolomics analysis by the high-performance liquid chromatography-tandem mass spectrometry technology, and different metabolites between normal human and the early stage pancreatic ductal adenocarcinoma patients are found. The different metabolites between normal human and pancreatic ductal adenocarcinoma patients are further analyzed by this technique to find the specific differentmetabolites C10:1 acyl carnitine and lysophosphatidyl choline LysoPC (14:0) of pancreatic ductal adenocarcinoma patients caused by cancer, i.e., the diagnostic molecules of the pancreatic ductal adenocarcinoma. According to the pancreatic ductal adenocarcinoma marker and the screening method thereof, the method can assist CA19-9 in diagnosing pancreatic duct adenocarcinoma patients, and can improve the diagnosis rate for the CA19-9 negative patients by 85%. The method is suitable for the screening of tumor markers.
Owner:HARBIN INST OF TECH
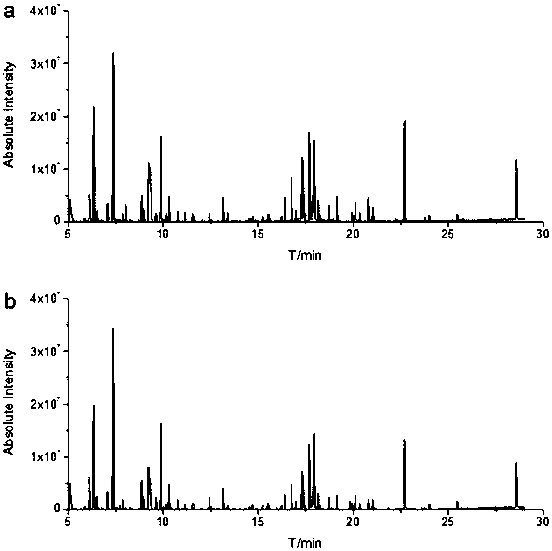
Absolute quantitative analysis method of lysophosphatidyl choline based on HPLC-MS/MS detection platform
ActiveCN107085061ACause interferenceExtended time intervalComponent separationInternal standardReserpine
The invention discloses an absolute quantitative analysis method of lysophosphatidyl choline based on an HPLC-MS / MS detection platform. The absolute quantitative analysis method establishes the HPLC-MS / MS detection platform, takes reserpine as an internal standard to draw the standard curves of LPC 14:0-LPC 18:0 serial substances, verifies the linearity, precision and accuracy of the detection platform, and detects the matrix effect of blood serum. Proven by verification, the HPLC-MS / MS detection platform established by the invention has higher stability and accuracy in detection of the LPC substances in the human blood serum, meets the clinical requirements and is suitable for clinical popularization.
Owner:TIANJIN CITY THIRD CENT HOSPITAL
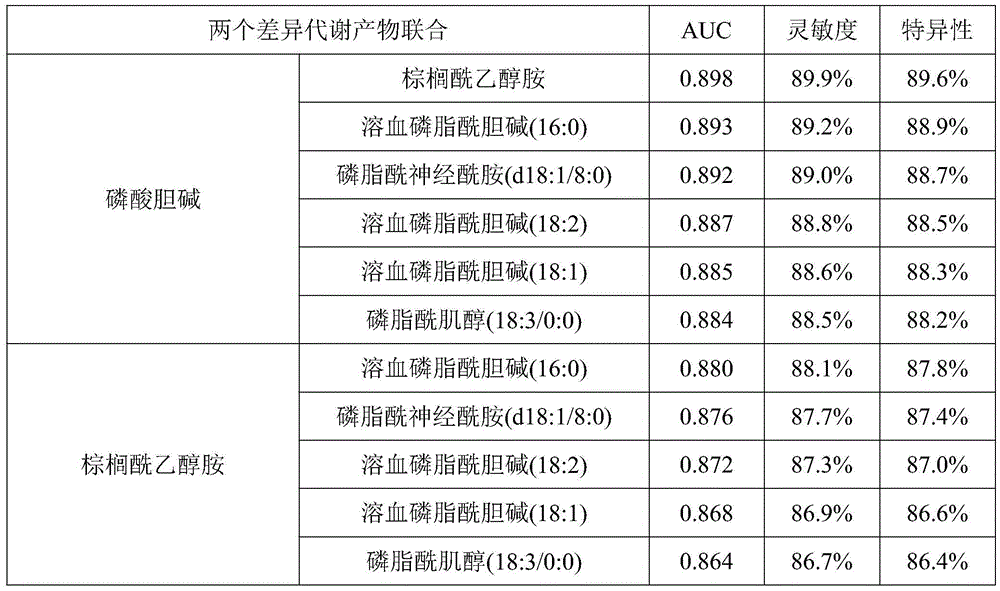
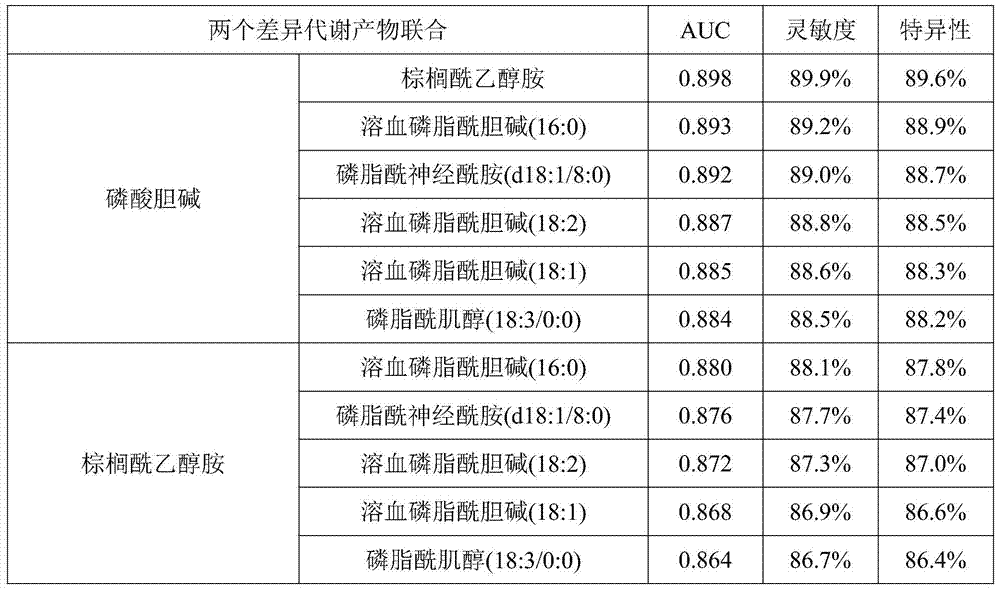
Metabolite marker for diagnosing and distinguishing coronary atherosclerosis and stable angina pectoris
ActiveCN105445408AEasy diagnosisImprove accuracyComponent separationBiological testingMetaboliteChenodeoxycholic acid
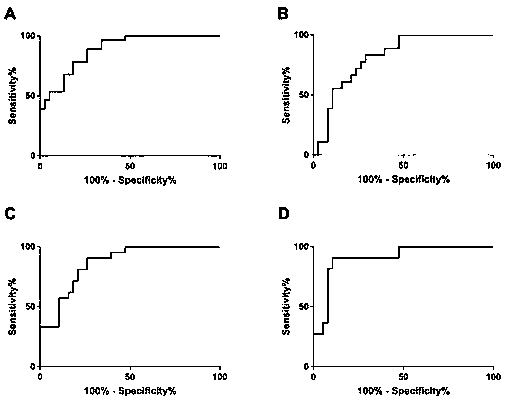
The invention discloses a metabolite marker for diagnosing and distinguishing coronary atherosclerosis and stable angina pectoris. The metabolite marker includes one or more of phosphorylcholine, palmitoylethanolamine, phytosphingosine, phosphatidylcholine, ethyl chenodeoxycholic acid, lysophosphatidyl choline (16:0), lysophosphatidyl choline (18:2) and phosphatidyl inositol (20:4 / 0:0). When the single metabolite marker is used for diagnosing and distinguishing a patient suffering from the stable angina pectoris and a patient suffering from the coronary atherosclerosis, the ROC areas under the curve (AUC) are greater than 0.7, and the marker has clinical diagnostic significance. When the markers are combined for diagnosis, the AUC is further improved with the increase of the combination number; when all eight markers are combined, the AUC is the highest and is up to 0.985, and the sensitivity and specificity are 97.4% and 98.0% respectively under the condition of an optimal cutoff value. The metabolite marker can accurately diagnose and distinguish the coronary atherosclerosis and the stable angina pectoris and is high in accuracy and good in sensitivity and specificity.
Owner:齐炼文
Plasma metabolism micromolecular markers related to lung cancer early diagnosis and application thereof
The invention discloses a group of plasma metabolism micromolecular markers related to lung cancer early diagnosis and an application thereof. The plasma metabolism micromolecular markers comprise oneor more of the following markers: propionic acid, aspartic acid, furan glucoside, citric acid, allose, talose, galactopyranose, pyruvic acid, tryptophan, leucyl proline, glyceryl phosphoryl choline,oleoyl carnitine, lysophosphatidyl choline (18:3), lysophosphatidyl choline (18:2), lysophosphatidyl choline (18:1), lysophosphatidyl choline (22:6), lysophosphatidyl ethanolamine (20:0), lysophosphatidyl ethanolamine (18:2), and complex aminoacyl valine. Compared with the traditional protein biomarkers, the group of the provided markers has the stronger outcome relevance with diseases, and is stable, minimally invasive, and easy to detect, capable of greatly improving the sensitivity and the specificity of the related disease diagnosis, and suitable for the early clinical diagnosis and monitoring of lung cancer.
Owner:CHINA PHARM UNIV
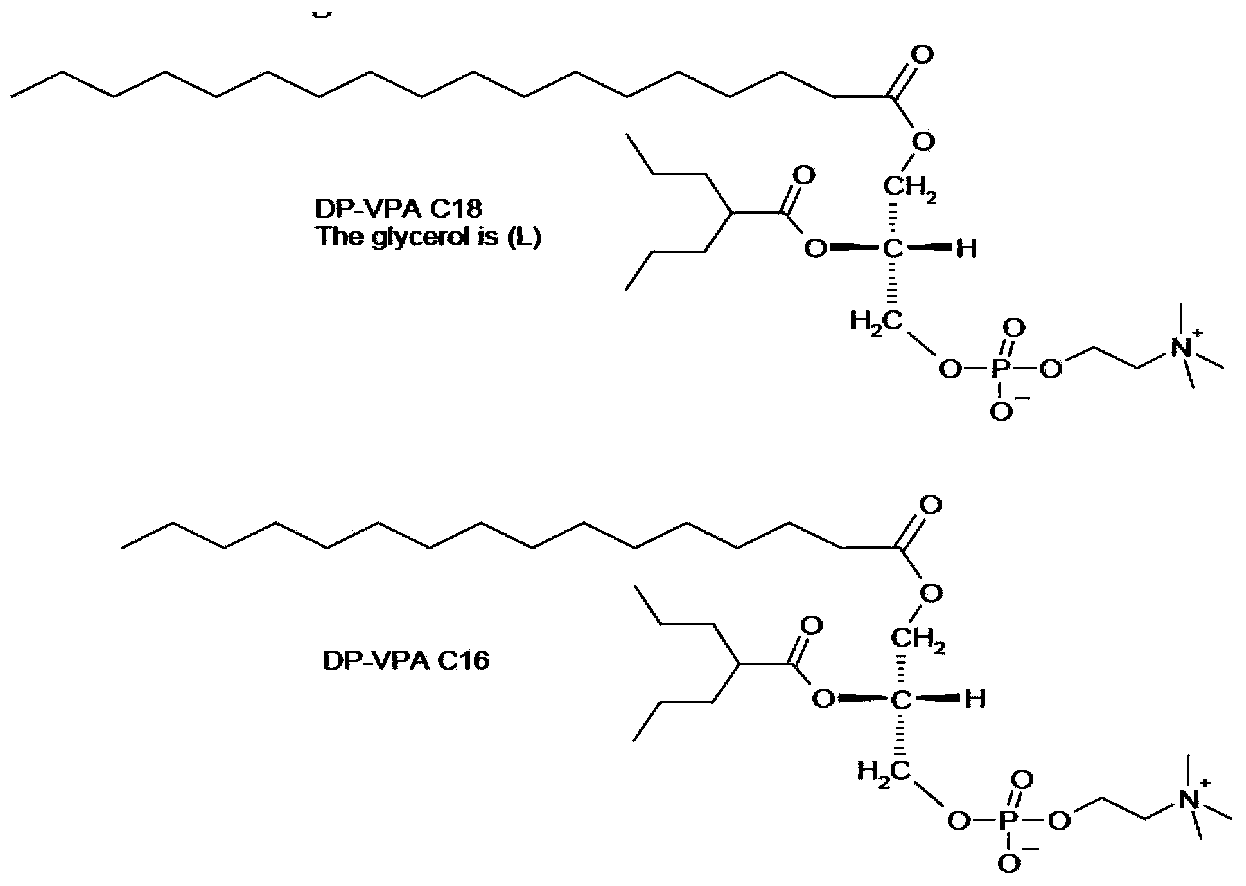
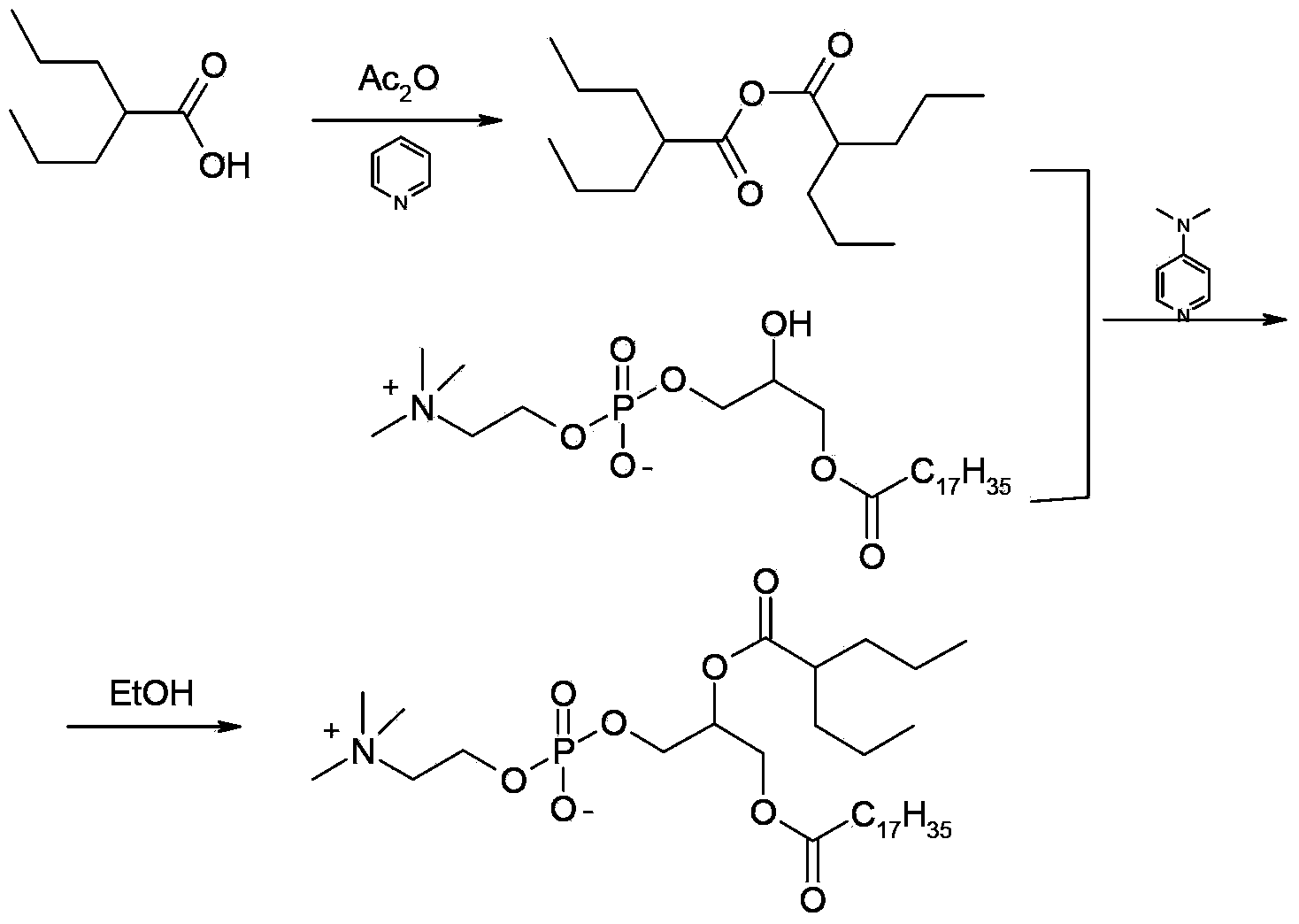
Preparation method of valproic acid phospholipid derivative
ActiveCN104230981AEasy to purifyHigh purityPhosphatide foodstuff compositionsAcetic anhydrideValproic Acid
The invention belongs to the field of organic compound preparation, and discloses a preparation method of a valproic acid phospholipid derivative. The method comprises the following steps: by using tetrahydrofuran as a solvent and triethylamine as an acid-binding agent, reacting propyl valeric chloride and equal mole of valproic acid at 10-50 DEG C, filtering, distilling to obtain valproic acid anhydride, adding lysophosphatidyl choline into the valproic acid anhydride solution, reacting at 60-85 DEG C under the catalytic action of 4-dimethylaminopyridine for 2-6 hours, adding acetone to precipitate the product, and refining with ethanol / acetone to obtain the valproic acid phospholipid derivative. By reacting the valproic acid and propyl valeric chloride to prepare the valproic acid anhydride, no influence of the acetic anhydride exists, and the valproic acid anhydride can be easily purified to obtain the high-purity valproic acid anhydride, thereby preparing the high-purity valproic acid phospholipid derivative.
Owner:NHWA PHARMA CORPORATION
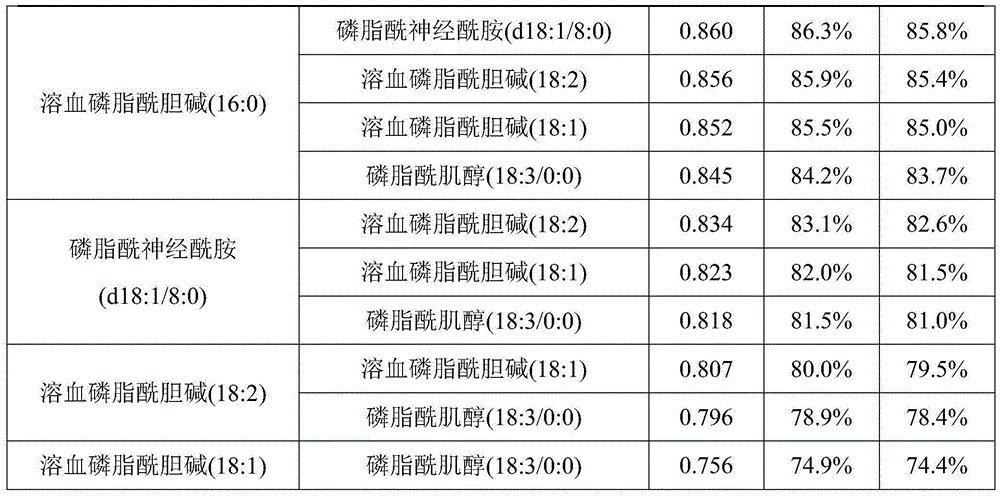
Metabolism marker for diagnosis of acute coronary syndrome
ActiveCN105486799AEasy diagnosisImprove accuracyComponent separationN-palmitoylethanolamineCoronary arteries
The invention discloses a metabolism marker for diagnosis of the acute coronary syndrome. The metabolism marker comprises one or more of phosphorylcholine, palmitoylethanolamine, lysophosphatidyl choline (16:0), phosphatidyl ceramide (d18:1 / 8:0), lysophosphatidyl choline (18:2), lysophosphatidyl choline (18:1) and phosphatidylinositol (18:3 / 0:0). When a single metabolism marker is used for diagnosis and distinguishing of acute coronary syndrome patients and coronary artery normal people, ROC area under the curve (AUC) is larger than 0.7 all the time, and clinical diagnosis significance is high; when several metabolism markers are combined to be used for diagnosis, AUC is further increased as the combination number increases and reaches the highest value 0.987 when all the seven metabolism markers are combined, and sensitivity and specificity are 99.1% and 98.9% respectively under the optimum cutoff value. The metabolism marker can be used for accurate diagnosis of the acute coronary syndrome and has high accuracy, sensitivity and specificity.
Owner:苏州帕诺米克生物科技有限公司
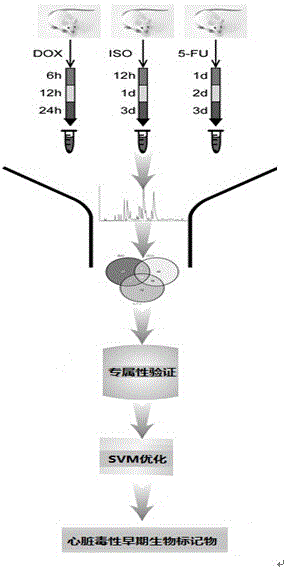
Application of endogenous small molecular substances to rapid detection of early cardiotoxicity
The invention discloses an application of endogenous small molecular substances to rapid detection of early cardiotoxicity, wherein the endogenous small molecular substances refers to nine early cardiotoxicity biomarkers: L-carnitine, L-tryptophan, L-palmitoyl carnitine, 19-hydroxyl deoxycorticosterone, cholic acid, lysophosphatidyl choline (14: 0), lysophosphatidyl choline (22: 6), lysophosphatidyl choline (20: 2) and lysophosphatidyl choline (16: 0), and also refers to four early cardiotoxicity exclusive biomarkers: L-carnitine, 19-hydroxyl deoxycorticosterone, lysophosphatidyl choline (14: 0) and lysophosphatidyl choline (20: 2). According to the application of the endogenous small molecular substances to the rapid detection of the early cardiotoxicity, the screened early cardiotoxicity biomarkers are capable of giving rapid alarms to the toxicity before the heart tissues suffer pathological damages, so that the toxicity discovery time is early than the conventional biochemical detection indexes, more flexible judgement is carried out on the drug toxicity and diseases, and more time is provided for the early discovery and early treatment of the clinic cardiotoxicity.
Owner:TIANJIN UNIV OF TRADITIONAL CHINESE MEDICINE
Composition for promoting coloring and sweetening of grapes
ActiveCN106614741AGreat tasteColoring condition improvedBiocidePlant growth regulatorsLysophosphatidyl ethanolamineMonopotassium phosphate
The invention discloses a composition for promoting coloring and sweetening of grapes. The composition comprises a component A (natural abscisic acid), a component B (monopotassium phosphate), a component C (one of lysophosphatidyl ethanolamine, lysophosphatidic acid and lysophosphatidyl choline) and a component D (one of sodium benzoate, levulinic acid and glutamic acid). A weight ratio of the component A to the component B to the component C to the component D is 1:6-30:0.04-2:1; the component A, the component B, the component C and the component D which are completely dissolved in absolute ethyl alcohol are mixed with distribution agents and water to obtain solution, and each liter of solution comprises 5-25 grams of component A, 30-150 grams of component B, 1-10 grams of component C, 5-25 grams of component D and 5 mL of distribution agents. The composition has the advantages that coloring of fruits can be obviously shifted to an earlier time after the composition is applied to the grapes, and the fruits are good in coloring uniformity; the sugar degree of the fruits can be upgraded, the sugar-acid ratios can be increased, and accordingly the quality can be improved; deterioration of the hardness of the fruits can be prevented after the composition is used, and accordingly the storage and transport quality of the grapes can be improved; the composition is safe and efficient in use.
Owner:LUDONG UNIVERSITY
Method for preparing phosphatidylcholine type omega-3 unsaturated fatty acid by using enzyme method
InactiveCN102181498AEffective absorptionMeet the requirementsFermentationDocosahexaenoic acidEicosapentaenoic acid
The invention relates to a method for preparing phosphatidylcholine type omega-3 unsaturated fatty acid by using an enzyme method. The method is characterized by comprising the following process steps of: 1, performing lipase selective enzymolysis, separation and purification to obtain high-purity seal oil free type omega-3 unsaturated fatty acid by taking seal oil triglyceride as a raw material;and 2, adding the high-purity seal oil free type omega-3 unsaturated fatty acid obtained in the step 1, serving as a raw material, into lysophosphatidyl choline to obtain a phosphatidylcholine type omega-3 unsaturated fatty product under the non-aqueous phase catalytic action of an enzyme. The method has the advantages of low cost and low pollution, and the prepared phosphatidylcholine type omega-3 unsaturated fatty acid has high purity and is rich in DPA (Docosaentaenoic Acid), EPA (Eicosapentaenoic Acid) and DHA (Docosahexaenoic Acid).
Owner:JIANGYIN BEC BIOSCI & TECH
Production method for lysophosphatidyl choline
InactiveCN106810574AReduce manufacturing costShort process routeGroup 5/15 element organic compoundsPhosphatide foodstuff compositionsGlycerolPhosphocholine
The invention provides a production method for lysophosphatidyl choline. By taking glycerol phosphocholine as a raw material, the lysophosphatidyl choline containing single ester group can be obtained by tin complexing and o-acylation; therefore, the production cost is low. The production method for the lysophosphatidyl choline, provided by the invention, has the advantages of short process route, simple treatment method and easy product purification.
Owner:HARBIN UNIV OF SCI & TECH
Method for preparing phosphatidylcholine type omega-3 unsaturated fatty acid by using enzyme method
InactiveCN102181498BEffective absorptionMeet the requirementsFermentationDocosahexaenoic acidEicosapentaenoic acid
The invention relates to a method for preparing phosphatidylcholine type omega-3 unsaturated fatty acid by using an enzyme method. The method is characterized by comprising the following process steps of: 1, performing lipase selective enzymolysis, separation and purification to obtain high-purity seal oil free type omega-3 unsaturated fatty acid by taking seal oil triglyceride as a raw material;and 2, adding the high-purity seal oil free type omega-3 unsaturated fatty acid obtained in the step 1, serving as a raw material, into lysophosphatidyl choline to obtain a phosphatidylcholine type omega-3 unsaturated fatty product under the non-aqueous phase catalytic action of an enzyme. The method has the advantages of low cost and low pollution, and the prepared phosphatidylcholine type omega-3 unsaturated fatty acid has high purity and is rich in DPA (Docosaentaenoic Acid), EPA (Eicosapentaenoic Acid) and DHA (Docosahexaenoic Acid).
Owner:JIANGYIN BEC BIOSCI & TECH
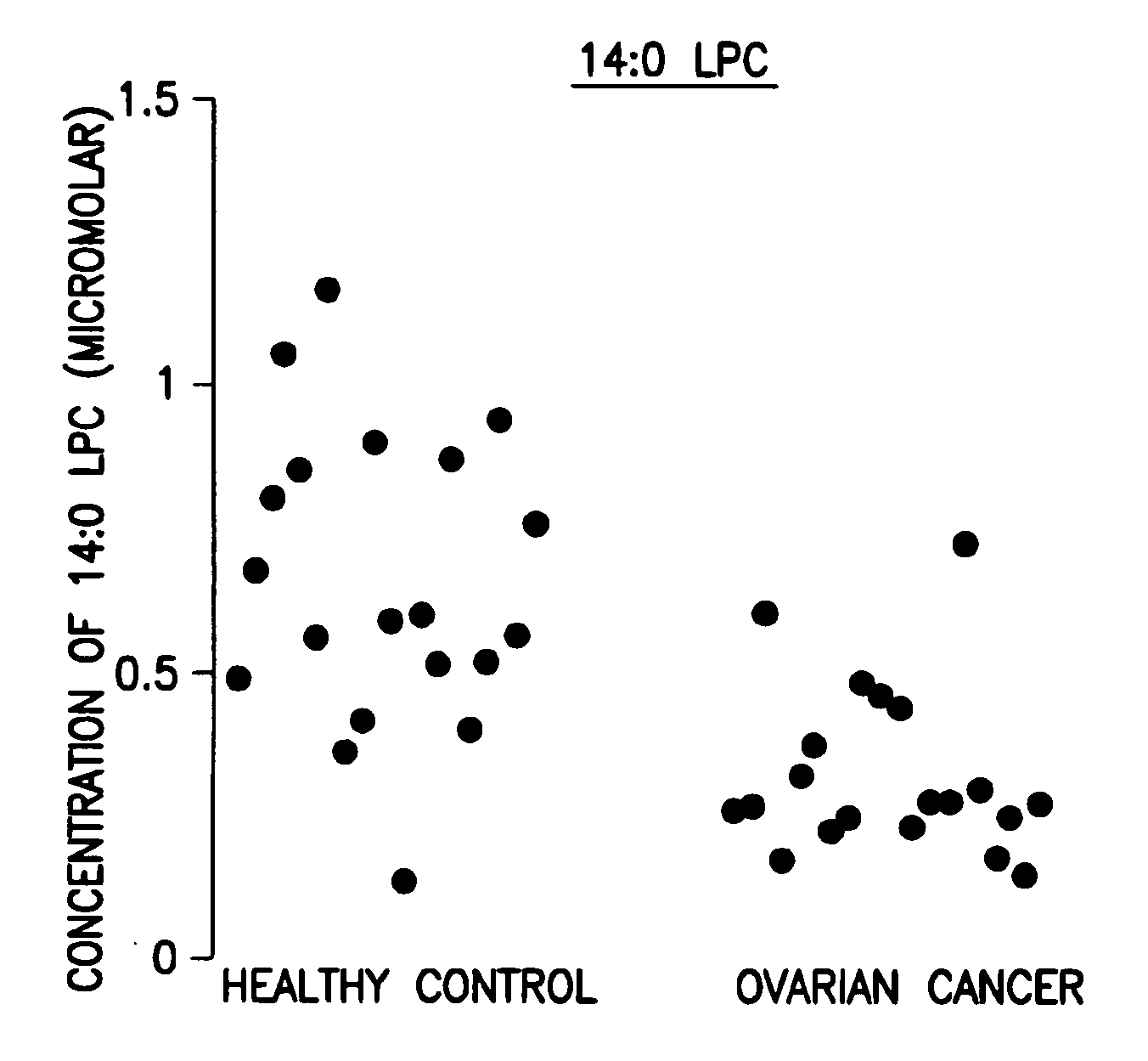
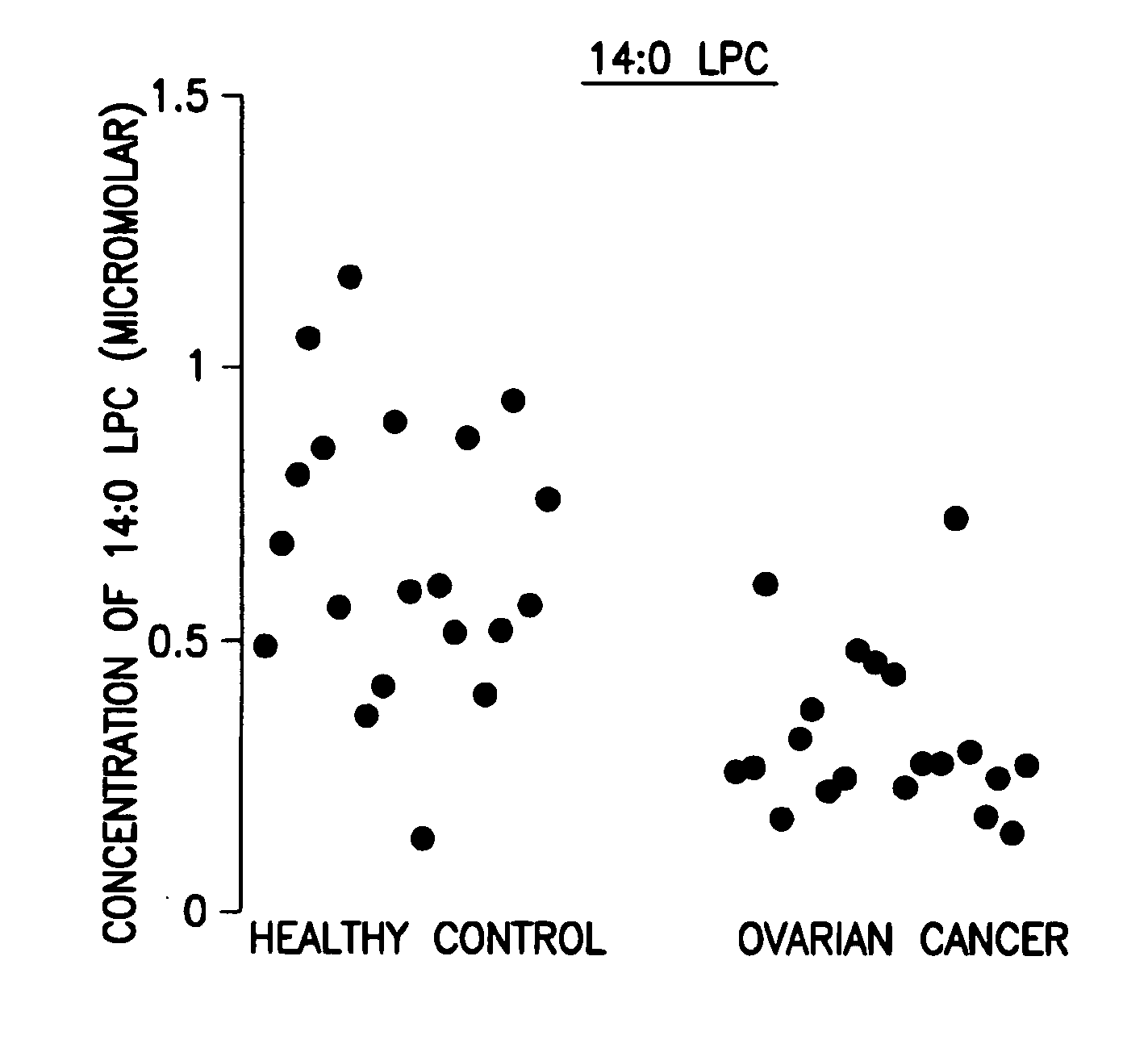
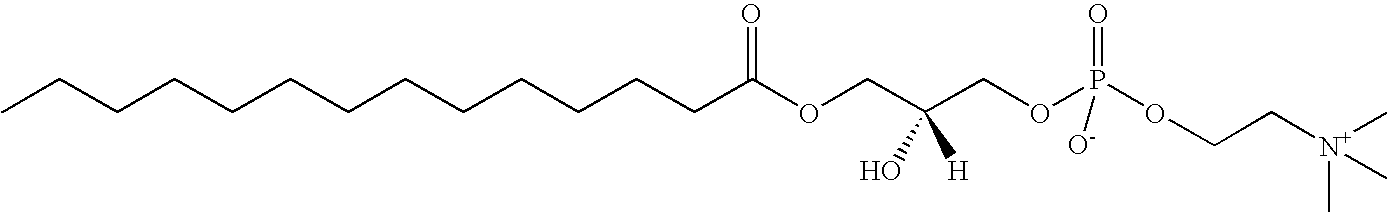
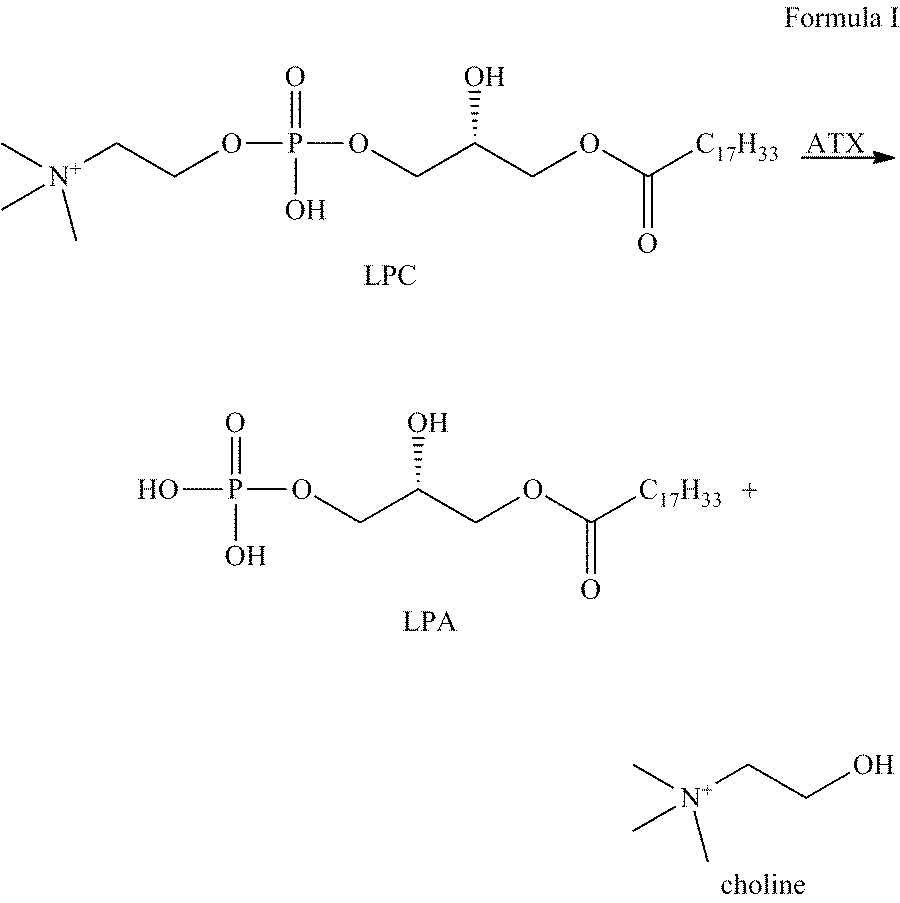
Methods for detecting or monitoring cancer using lpc as a marker
A method of detecting a cancer, such as ovarian cancer, in a test subject including (a) determining the amount of a lysophosphatidyl choline in a sample of a bodily fluid taken from the test subject, and (b) comparing the amount of the lysophosphatidyl choline in the sample of the bodily fluid taken from the test subject to a range of amounts of lysophosphatidyl choline found in samples of the bodily fluid taken from a group of normal subjects of the same species as the test subject and lacking the cancer, such as ovarian cancer, whereby a change in the amount of the lysophosphatidyl choline in the sample of the bodily fluid taken from the test subject indicates the presence of the cancer, such as ovarian cancer.
Owner:H LEE MOFFITT CANCER CENT & RES INST INC +1
A composition for promoting coloring and sweetening of grapes
ActiveCN106614741BGreat tasteColoring condition improvedPlant growth regulatorsBiocideLysophosphatidyl ethanolaminePropanoic acid
The invention discloses a composition for promoting coloring and sweetening of grapes. The composition comprises a component A (natural abscisic acid), a component B (monopotassium phosphate), a component C (one of lysophosphatidyl ethanolamine, lysophosphatidic acid and lysophosphatidyl choline) and a component D (one of sodium benzoate, levulinic acid and glutamic acid). A weight ratio of the component A to the component B to the component C to the component D is 1:6-30:0.04-2:1; the component A, the component B, the component C and the component D which are completely dissolved in absolute ethyl alcohol are mixed with distribution agents and water to obtain solution, and each liter of solution comprises 5-25 grams of component A, 30-150 grams of component B, 1-10 grams of component C, 5-25 grams of component D and 5 mL of distribution agents. The composition has the advantages that coloring of fruits can be obviously shifted to an earlier time after the composition is applied to the grapes, and the fruits are good in coloring uniformity; the sugar degree of the fruits can be upgraded, the sugar-acid ratios can be increased, and accordingly the quality can be improved; deterioration of the hardness of the fruits can be prevented after the composition is used, and accordingly the storage and transport quality of the grapes can be improved; the composition is safe and efficient in use.
Owner:LUDONG UNIVERSITY
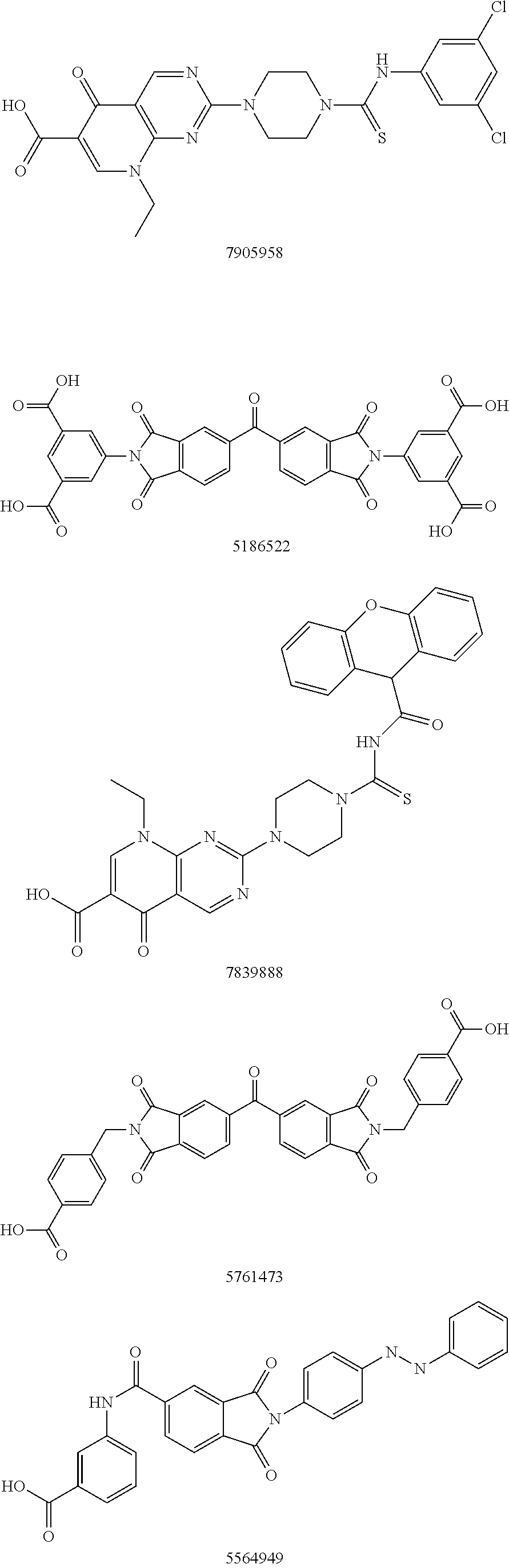
Autotaxin inhibitors
ActiveUS8969590B1Reduce conversionReadily availableMonoazo dyesOrganic chemistryAutotaxinReactive site
Classes of compounds that exhibit effective inhibition of autotaxin enzymes are provided. Such classes include thioureas, diphenyldiazerenes, xanthenes, and isoindoles and exhibit reactivity with autotaxin to ultimately reduce the size of the reactive sites thereon to prevent conversion of lysophosphatidyl choline to lysophophatidic acid. Furthermore, such compounds can be incorporated within delivery forms for human ingestion. As such, these compounds accord an excellent manner of potentially reducing generation of certain cancers attributable to the presence of naturally occurring autotaxin within the human body. Methods of inactivating autotaxin to certain degrees therewith such compounds are encompassed within invention as well.
Owner:UNIVERSITY OF MEMPHIS RESEARCH FOUNDATION
Metabolic markers for the diagnosis of acute coronary syndrome
The invention discloses a metabolism marker for diagnosis of the acute coronary syndrome. The metabolism marker comprises one or more of phosphorylcholine, palmitoylethanolamine, lysophosphatidyl choline (16:0), phosphatidyl ceramide (d18:1 / 8:0), lysophosphatidyl choline (18:2), lysophosphatidyl choline (18:1) and phosphatidylinositol (18:3 / 0:0). When a single metabolism marker is used for diagnosis and distinguishing of acute coronary syndrome patients and coronary artery normal people, ROC area under the curve (AUC) is larger than 0.7 all the time, and clinical diagnosis significance is high; when several metabolism markers are combined to be used for diagnosis, AUC is further increased as the combination number increases and reaches the highest value 0.987 when all the seven metabolism markers are combined, and sensitivity and specificity are 99.1% and 98.9% respectively under the optimum cutoff value. The metabolism marker can be used for accurate diagnosis of the acute coronary syndrome and has high accuracy, sensitivity and specificity.
Owner:苏州帕诺米克生物科技有限公司
Method for preparing lysophosphatidyl choline by enzymatic alcoholysis
Owner:HENAN UNIVERSITY OF TECHNOLOGY
Application of endogenous small molecule substances in rapid detection of early cardiotoxicity
The invention discloses an application of endogenous small molecular substances to rapid detection of early cardiotoxicity, wherein the endogenous small molecular substances refers to nine early cardiotoxicity biomarkers: L-carnitine, L-tryptophan, L-palmitoyl carnitine, 19-hydroxyl deoxycorticosterone, cholic acid, lysophosphatidyl choline (14: 0), lysophosphatidyl choline (22: 6), lysophosphatidyl choline (20: 2) and lysophosphatidyl choline (16: 0), and also refers to four early cardiotoxicity exclusive biomarkers: L-carnitine, 19-hydroxyl deoxycorticosterone, lysophosphatidyl choline (14: 0) and lysophosphatidyl choline (20: 2). According to the application of the endogenous small molecular substances to the rapid detection of the early cardiotoxicity, the screened early cardiotoxicity biomarkers are capable of giving rapid alarms to the toxicity before the heart tissues suffer pathological damages, so that the toxicity discovery time is early than the conventional biochemical detection indexes, more flexible judgement is carried out on the drug toxicity and diseases, and more time is provided for the early discovery and early treatment of the clinic cardiotoxicity.
Owner:TIANJIN UNIV OF TRADITIONAL CHINESE MEDICINE
A pancreatic ductal adenocarcinoma marker and its screening method
ActiveCN109239210BImprove diagnosis rateAccurately reflect differences in metabolic profilesComponent separationPancreas Ductal AdenocarcinomaMetabolite
The invention discloses a pancreatic ductal adenocarcinoma marker and a screening method thereof, which belongs to the field of clinical test and diagnosis. In view of the problem that the detection sensitivity and the detection specificity of the existing pancreatic ductal adenocarcinoma diagnosis marker are poor, serum of an early patient of early stage pancreatic duct adenocarcinoma is subjected to a trace metabolomics analysis by the high-performance liquid chromatography-tandem mass spectrometry technology, and different metabolites between normal human and the early stage pancreatic ductal adenocarcinoma patients are found. The different metabolites between normal human and pancreatic ductal adenocarcinoma patients are further analyzed by this technique to find the specific differentmetabolites C10:1 acyl carnitine and lysophosphatidyl choline LysoPC (14:0) of pancreatic ductal adenocarcinoma patients caused by cancer, i.e., the diagnostic molecules of the pancreatic ductal adenocarcinoma. According to the pancreatic ductal adenocarcinoma marker and the screening method thereof, the method can assist CA19-9 in diagnosing pancreatic duct adenocarcinoma patients, and can improve the diagnosis rate for the CA19-9 negative patients by 85%. The method is suitable for the screening of tumor markers.
Owner:HARBIN INST OF TECH
Self-Emulsifying Bioactive Concentrates
A self-emulsifying bioactive concentrate produced by a process comprising the steps of (a) creating a lysophospholipid concentrate comprised of a de-oiled lecithin source and 0.1% to 10% of an enzyme with having phospholipase A activity, wherein the lysophospholipid concentrate contains greater than 20% lysophosphatidyl-choline; and (b) combining the lysophospholipid concentrate from step (a) with a retinyl ester formed by the reaction of retinol and one or a mixture of unsaturated fatty acid(s).
Owner:OWEN BIOSCIENCES INC
Application of a group of plasma metabolic small molecule markers related to early diagnosis of lung cancer
ActiveCN108414660BIncreased sensitivityImprove featuresComponent separationDiseaseLysophosphatidyl ethanolamine
Owner:CHINA PHARM UNIV
Medicament for treating tumors
InactiveCN101979090BMitigation of lethalityIncrease lethalityPeptide/protein ingredientsAmide active ingredientsAutotaxinTumor therapy
Owner:BEIJING NORMAL UNIVERSITY
A kind of preparation method of polyene phosphatidylcholine for injection
ActiveCN104592292BAchieve decolorizationTo achieve impurity removalPhosphatide foodstuff compositionsTG - TriglycerideSolvent
The invention discloses a preparation method for polyene phosphatidyl choline for injection. The preparation method comprises the following steps: dissolving with a proper solvent by taking soyabean lecithin as a raw material, performing elution twice with a ternary mixed solvent consisting of multi-halogenated hydrocarbon, low carbon alcohol and water through column chromatography, and drying to obtain a product polyene phosphatidyl choline. The product obtained by the preparation method disclosed by the invention is high in phosphatidylcholine content (98.0%-99.9%, HPLC (high performance liquid chromatography) process), low in impurity content (less than 1.0% of lysophosphatidyl choline, less than 0.5% of phosphatidyl inositol, less than 0.5% of phosphatidyl ethanolamine, less than 0.2% of free fatty acid and less than 0.2% of triglyceride), and low in oxidative index (an acid value lower than 0.5, a peroxide value lower than 1.0 and methoxy phenylamine value lower than 5.0), so that the product quality reaches the quality standards of polyene phosphatidyl choline for injection.
Owner:GUANGZHOU HANFANG PHARMA
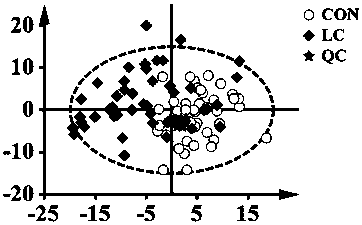
Target lipidomics method based on modelling-predicting strategy
ActiveCN109725046ACreate an analysis methodMaterial analysis by electric/magnetic meansRetention timeCarbon chain
The invention belongs to the technical field of medicines, in particular relates to a target lipidomics method, and, more exactly, relates to high-efficiency and sensitive qualitative and quantitativeanalysis onto lysophosphatidyl choline (LPC) in plasma by utilization of a modelling-predicting strategy. According to the target lipidomics method in the invention, a multiple linear regression model is established by taking the carbon chain length (x1) and the double-bond number (x2) as independent variables, and the declustering potential (DP), the collision energy (CE), the retention time (RT) and the response factor (RF) as dependent variables; liquid mass spectrometry parameter rules of LPC(13:0), LPC(14:0), LPC(15:0), LPC(17:0), LPC(18:0), LPC(19:0) and LPC(20:0) reference substances are summarized; then, corresponding parameters that the LPC of the reference substance cannot be obtained are predicted by utilization of a equation; quantitative analysis of LPC in the plasma is carried out by utilization of the predicted liquid mass spectrometry parameters; biomarkers of different cancers are screened through independent sample T check, PLS-DA and a single-factor ROC curve; and furthermore, the diagnostic capability is evaluated through a multi-factor ROC curve.
Owner:SHENYANG PHARMA UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com