Absolute quantitative analysis method of lysophosphatidyl choline based on HPLC-MS/MS detection platform
A technology for quantitative analysis of phosphatidylcholine, applied in the field of medical testing, can solve the problems that LPC substances cannot be popularized, unrealistic, unsatisfactory results of quality control and performance verification, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Embodiment 1 HPLC-MS / MS methodology establishment
[0056] 1. Reagents and instruments
[0057] The sample analysis process mainly involves sample pretreatment, chromatographic separation, and mass spectrometry identification, and the reagents and instruments used are mainly required in these three steps.
[0058] 1.1 Reagents and instruments required for specimen pretreatment
[0059] Reagents: (1) Methanol (manufactured by Honeywell, LC-MS grade in purity), (2) 2,6-di-tert-butyl-p-cresol (2,6-di-tert-butyl-p-cresol also known as ButylatedHydroxytoluene , BHT), the purity of this reagent is superior grade, produced by Bailingwei Enterprise Co., Ltd.
[0060] Instrument: Cleanert PPT 96-well protein precipitation plate, catalog number 96CD2025Q; Cleanert M96 biological sample pretreatment instrument; V96 sample nitrogen blowing concentrator and 96-well collection plate. The above instruments are all from Tianjin Bona Aegel Technology Co., Ltd.
[0061] 1.2 Rea...
Embodiment 2
[0088] Example 2 HPLC-MS / MS Methodology Verification
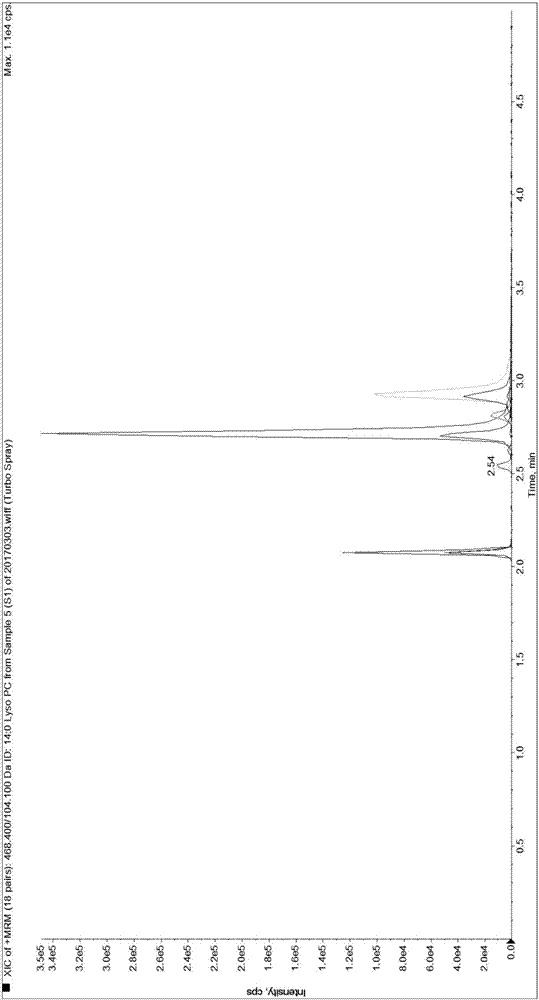
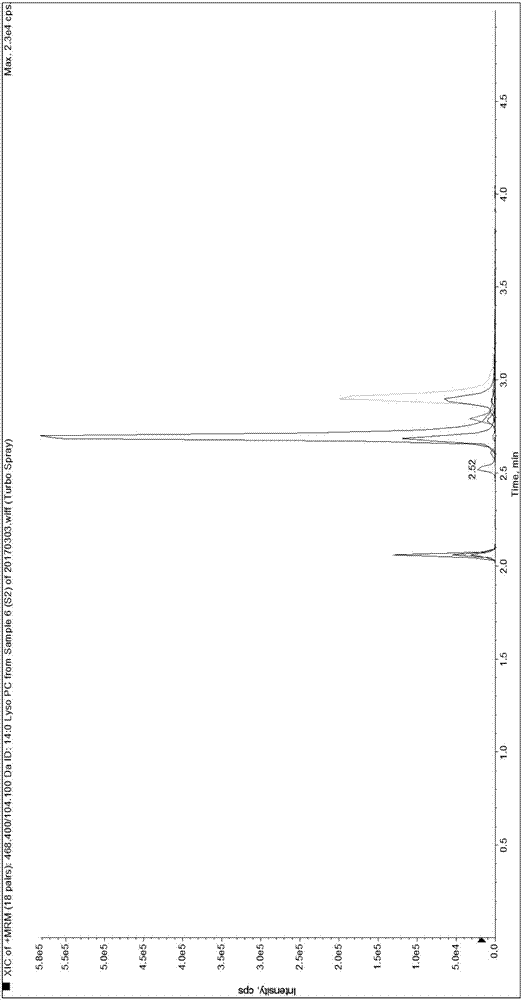
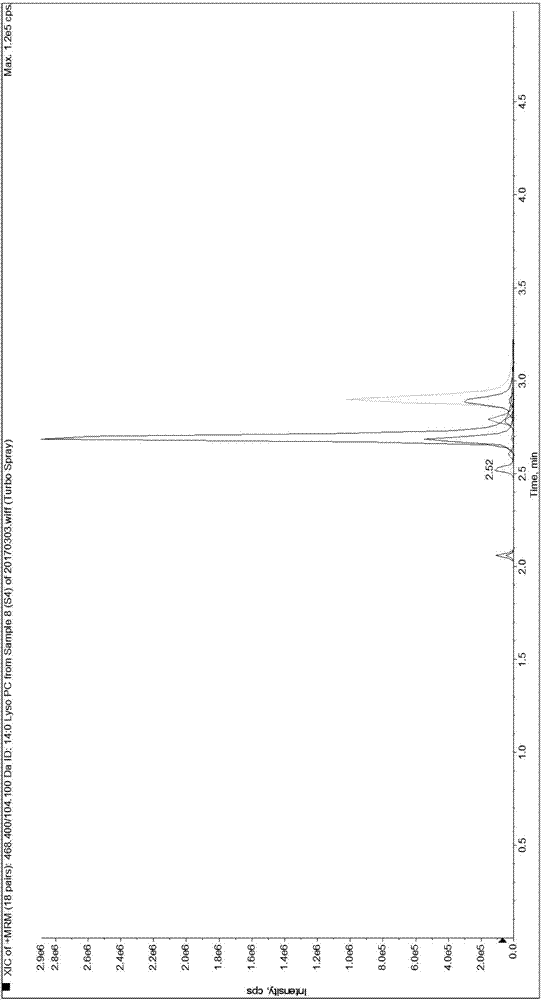
[0089] Since it is difficult to obtain a matrix completely free of LPC 14:0, LPC 15:0, LPC 16:0, LPC 17:0 and LPC 18:0, the present invention first uses 0.9% normal saline instead of human serum for methodological evaluation and performance verification.
[0090] 1. Verification method
[0091] (1) Evaluation of linear relationship and sensitivity
[0092] Weigh 1.875mg, 3.750mg, 56.250mg, 5.625mg and 37.500mg of LPC 14:0, LPC 15:0, LPC 16:0, LPC 17:0 and LPC 18:0 standards and dissolve them in 50mL methanol solution , take 5 μL and transfer it to 20 μL methanol solution, that is, make the standard curve sample of S7 concentration in Table 3, and use the standard curve sample of S7 concentration to dilute the standard curve sample of each gradient concentration of S1-S6 for standard curve determination and Linear relationship verification. Dilute the standard curve sample of S1 concentration to a lower concentration ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com