Novel serum metabolite composition and application thereof as liver cancer diagnosis marker
A technology of metabolites and serum, applied in the field of analytical chemistry and clinical medicine, can solve the problems of lack of early warning value, low diagnostic sensitivity and specificity, and limited inspection effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
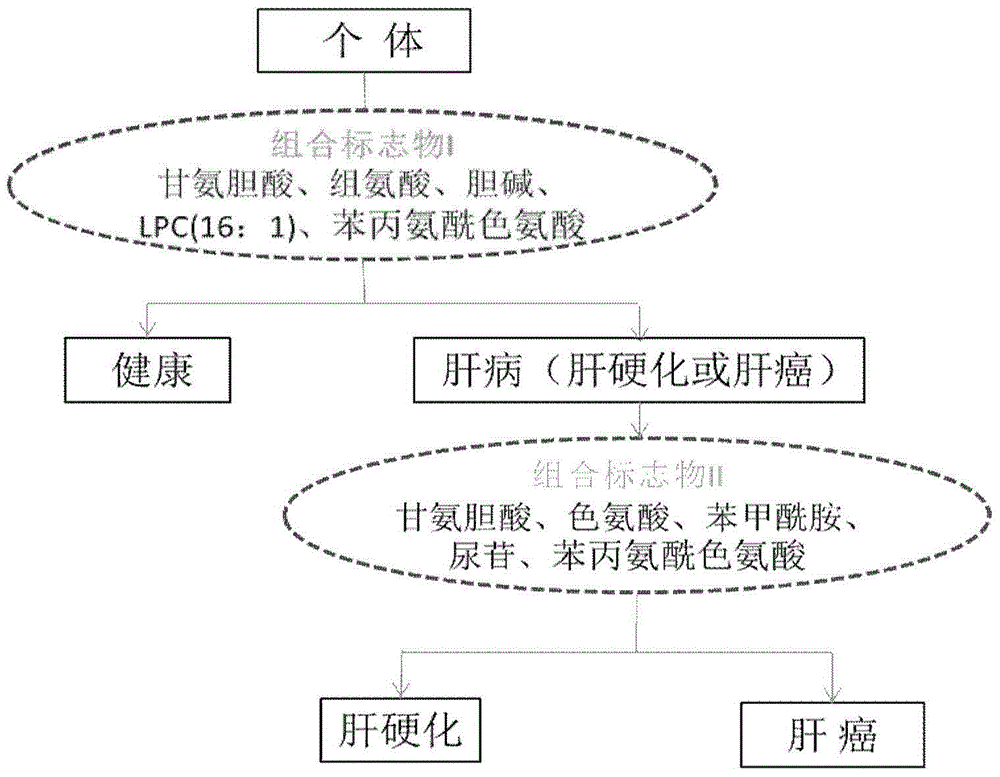
[0086] 1. Serum sample collection Before the collection, the included volunteers signed the informed consent.
[0087] Inclusion criteria for normal control, liver cirrhosis and primary liver cancer groups: refer to "Huang Jiasi Surgery (7th Edition)" People's Health Publishing House, Beijing, 2008
[0088] Inclusion criteria for the healthy control group: normal physical examination population (between 40 and 70 years old). Physical examination of healthy people.
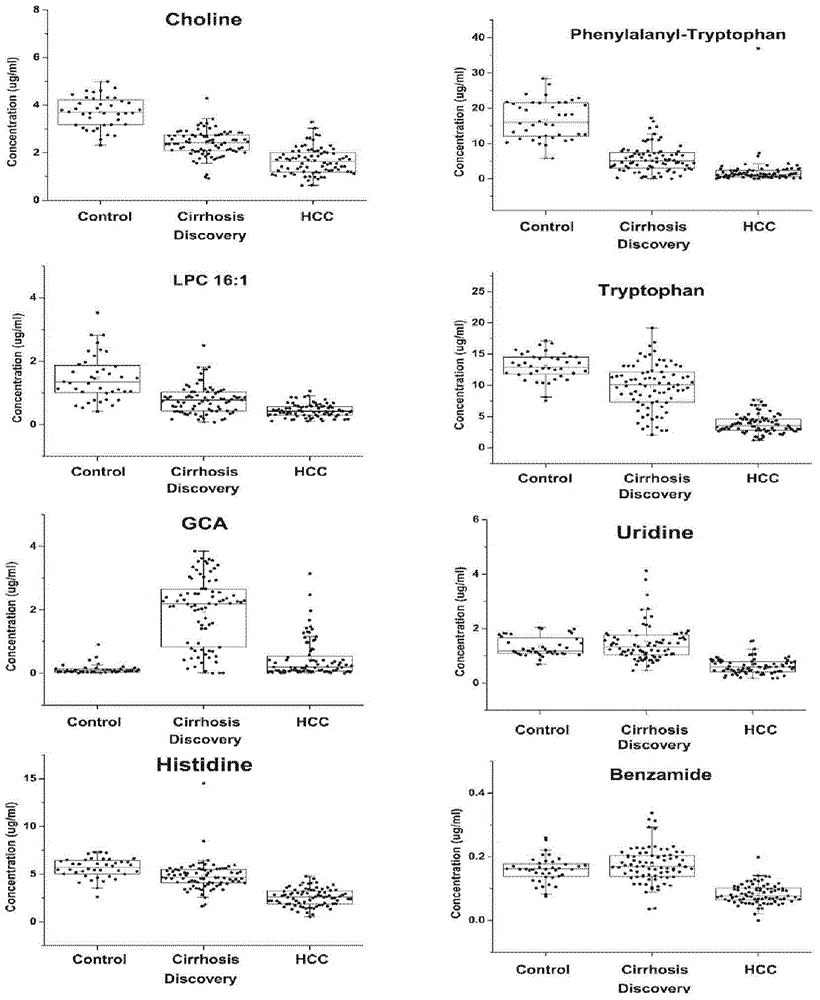
[0089] Serum samples were used under the same conditions: serum samples from 40 normal persons, 79 cases of liver cirrhosis and 80 cases of liver cancer; within 24 hours after admission, 4ml of fasting anticoagulant blood and 4ml of procoagulant blood were collected, and the serum was separated within half an hour. Store in -80°C refrigerator for future inspection.
[0090] 2. Analysis method
[0091] 2.1 Serum sample pretreatment
[0092]Thaw blood samples at 4°C, take 80 μL of serum samples, add 320 μL of ace...
Embodiment 2
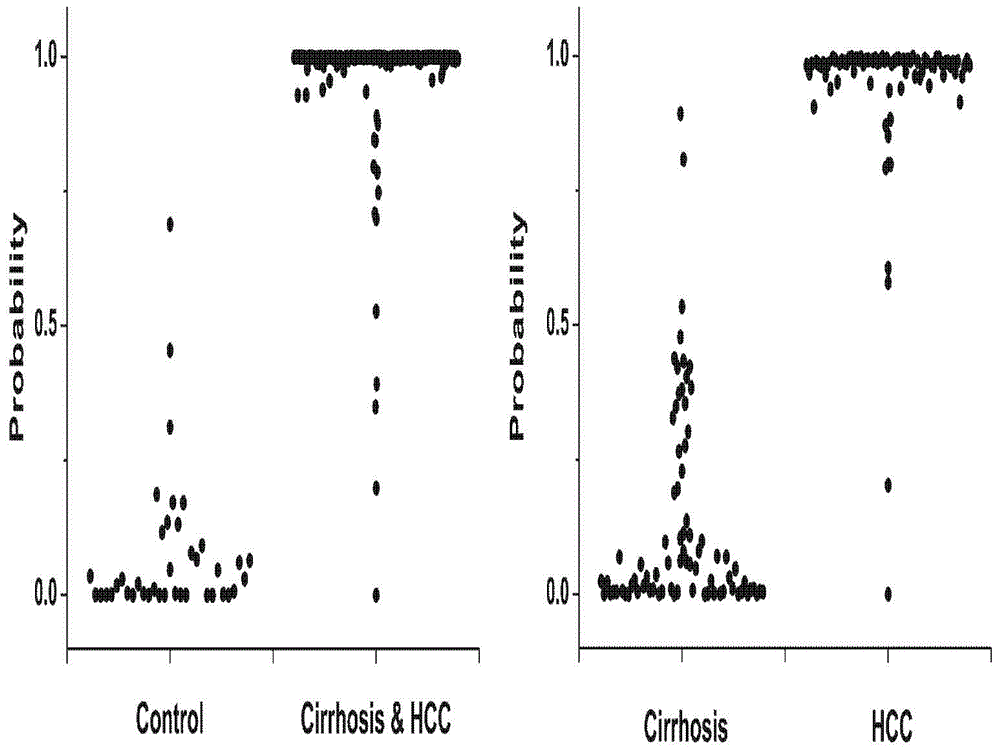
[0106] In order to verify the stability and reliability of the combined markers for the diagnosis of early liver cancer, small liver cancer samples with small tumor diameters were added for verification. The analytical method, instrument and reagent used in this experiment are all completely consistent with those in Example 1.
[0107] 1. Serum sample collection Before the collection, the included volunteers signed the informed consent.
[0108] Inclusion criteria for normal control, liver cirrhosis and primary liver cancer groups: refer to "Huang Jiasi Surgery (7th Edition)" People's Health Publishing House, Beijing, 2008
[0109] Inclusion criteria for the healthy control group: normal physical examination population (between 40 and 70 years old). Physical examination of healthy people.
[0110] Serum samples were used under the same conditions: 40 cases of normal people, 65 cases of liver cirrhosis, 75 cases of small liver cancer patients and 29 cases of large liver cance...
Embodiment 3
[0122] In order to verify the stability and reliability of the combined markers for the diagnosis of early liver cancer, liver cancer patients from different regions than those in Examples 1 and 2 were added to carry out the verification experiment II. The analytical method, instrument and reagent used in this experiment are all completely consistent with those in Example 1.
[0123] 1. Serum sample collection Before the collection, the included volunteers signed the informed consent.
[0124] Inclusion criteria for normal control, liver cirrhosis and primary liver cancer groups: refer to "Huang Jiasi Surgery (7th Edition)" People's Health Publishing House, Beijing, 2008
[0125] Inclusion criteria for the healthy control group: normal physical examination population (between 40 and 70 years old). Physical examination of healthy people.
[0126] Serum samples were used under the same conditions: serum samples from 31 normal persons, 48 cases of liver cirrhosis, and 92 case...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com