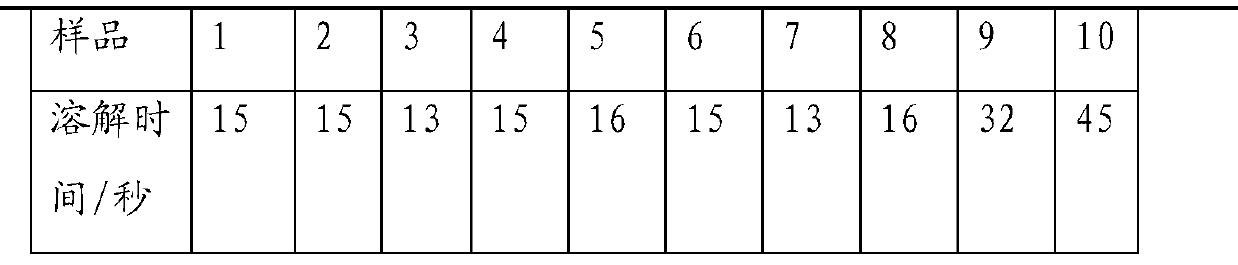
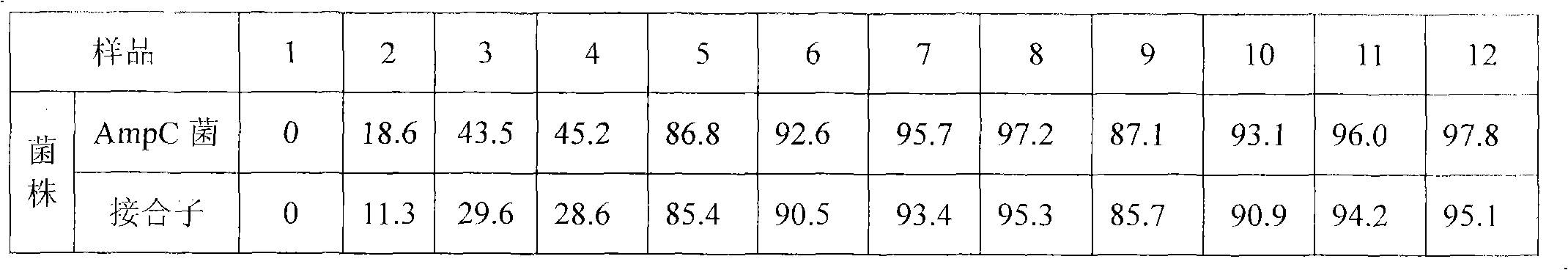
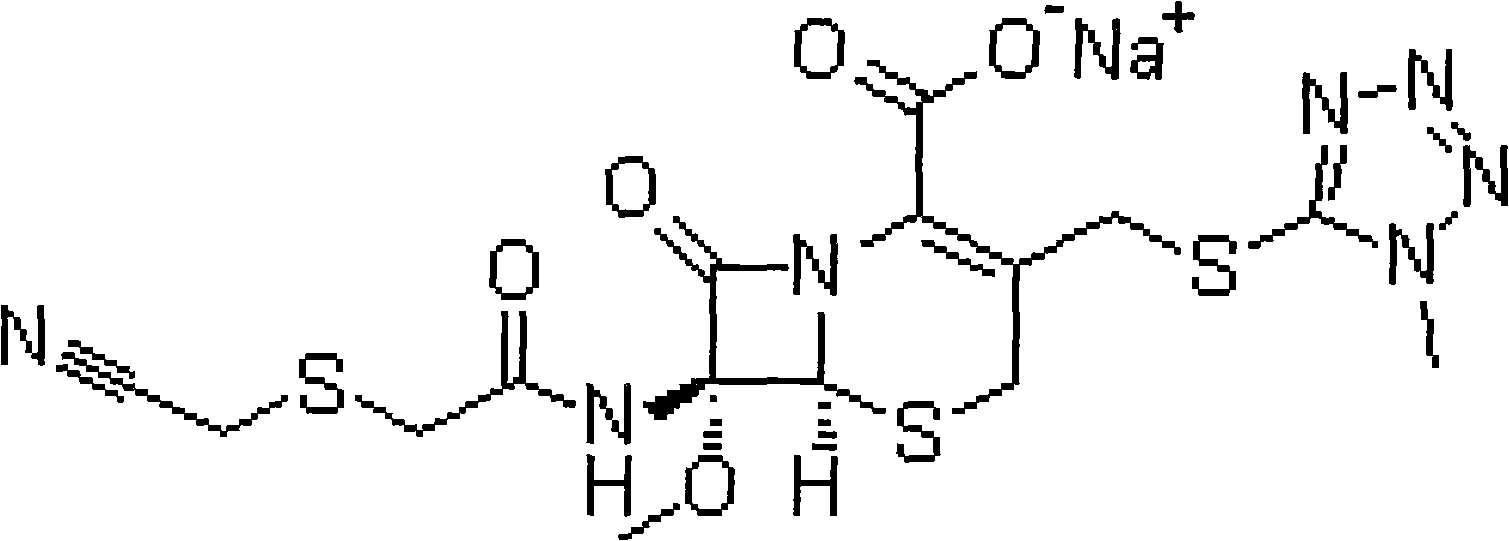
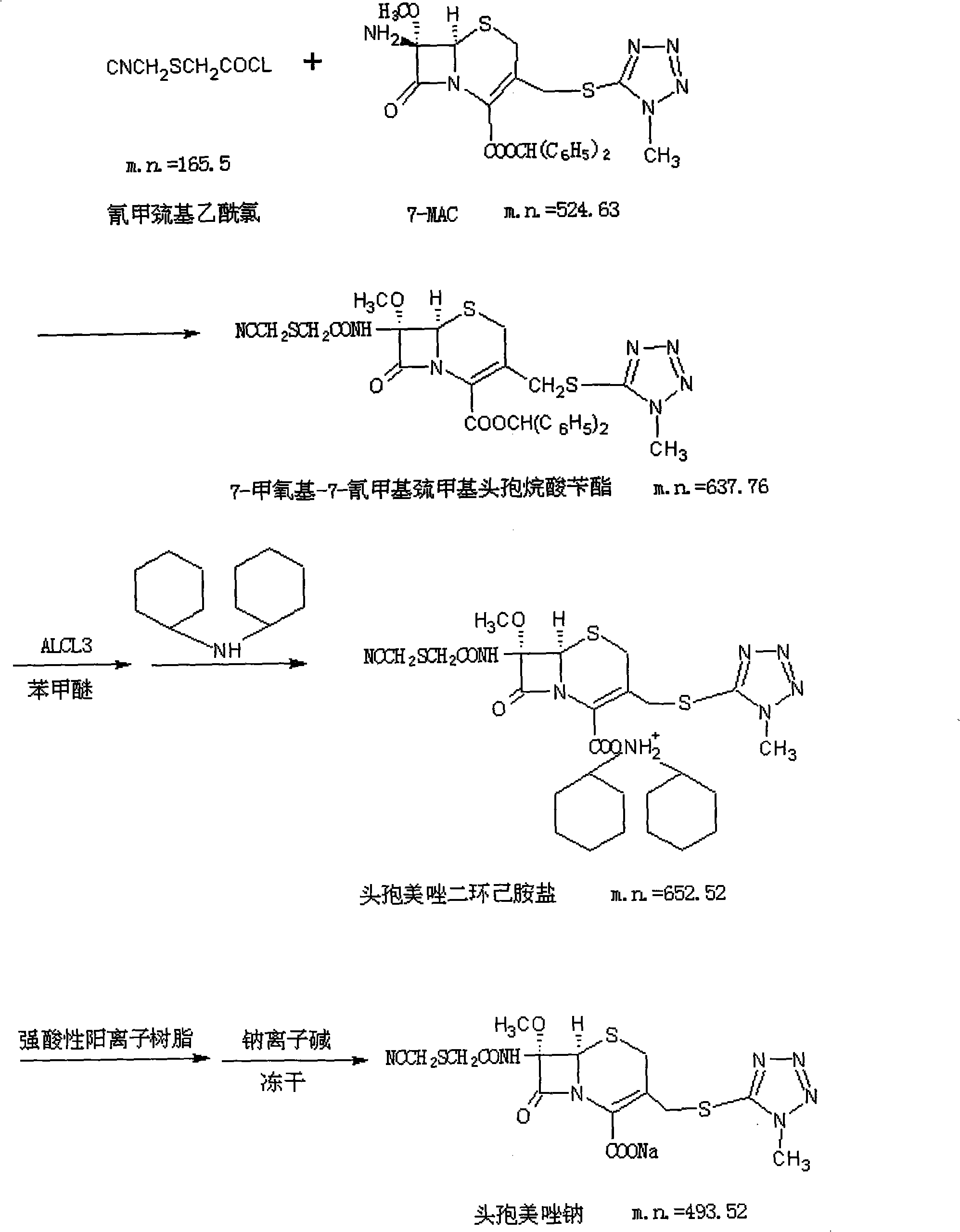
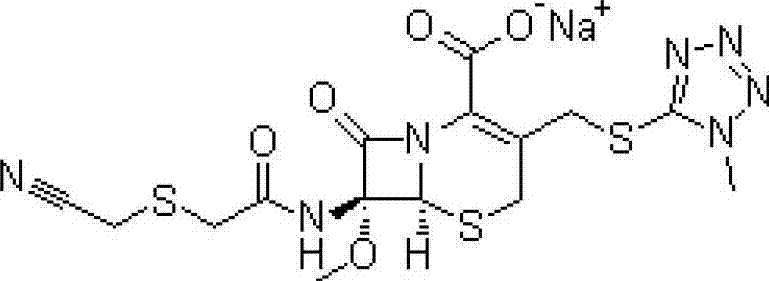
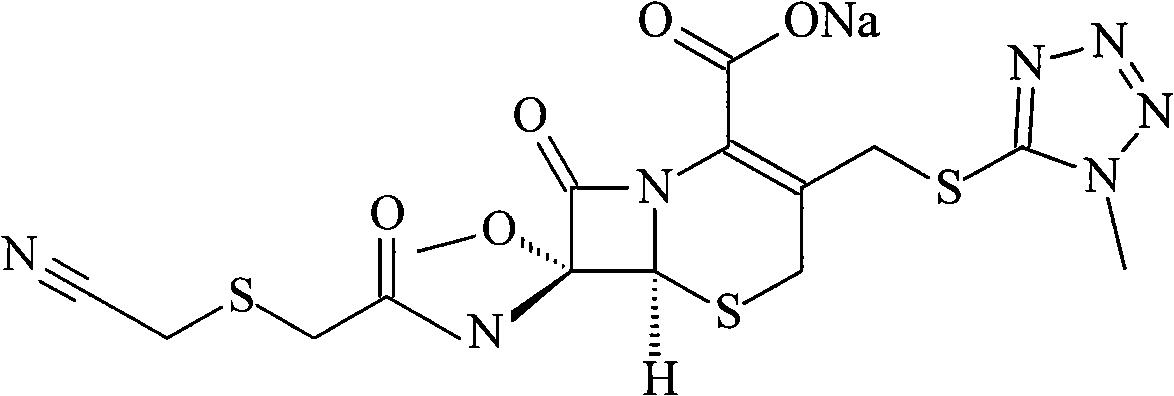
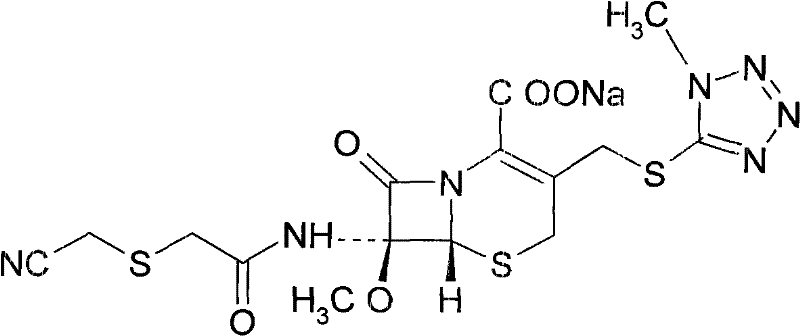
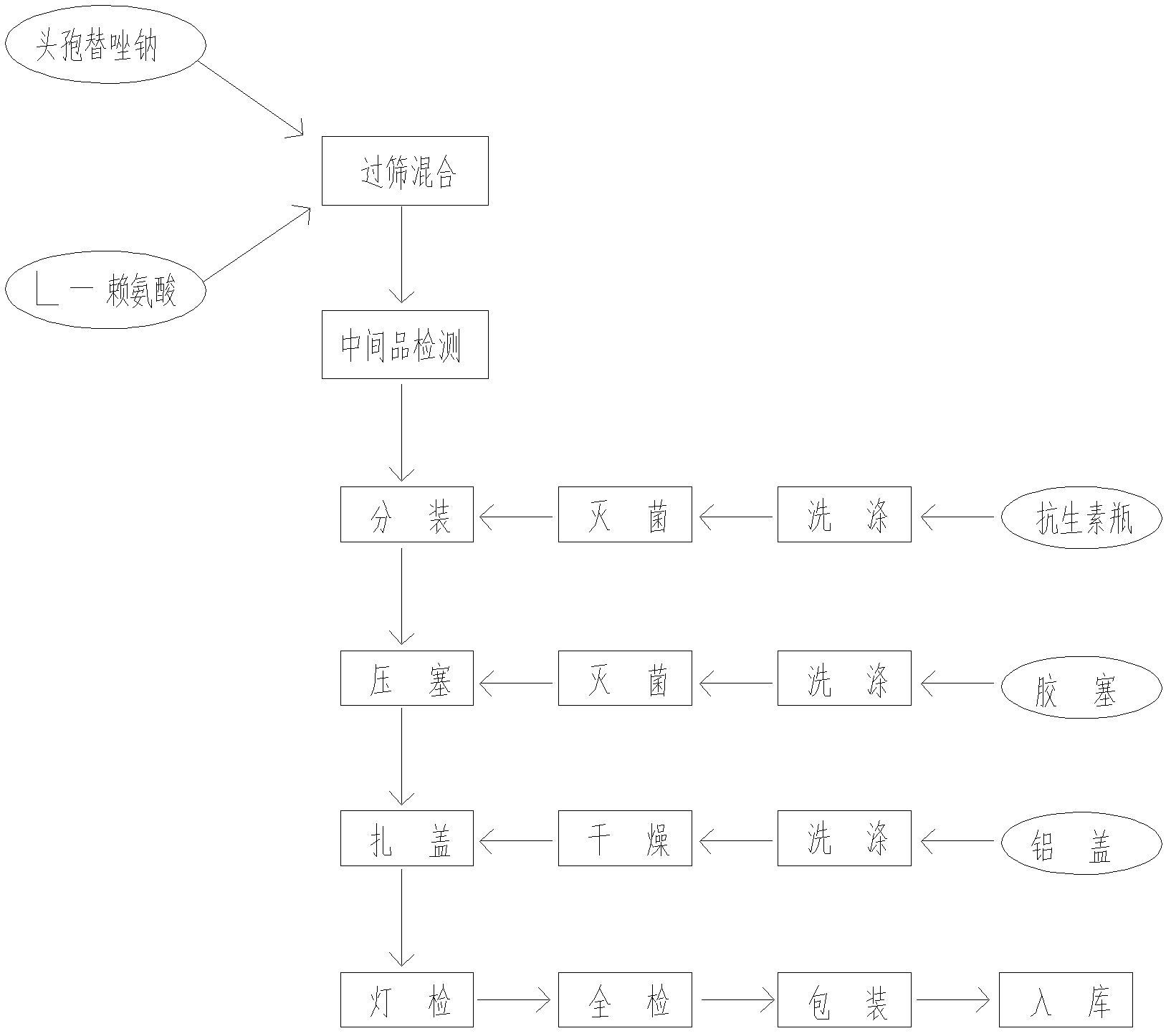Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
60 results about "CEFMETAZOLE SODIUM" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for preparing powder injection using superfine communication technique and prepared products
InactiveCN101332188AReduce contentUniform colorAntibacterial agentsOrganic active ingredientsLatamoxefClindamycin Phosphate
The present invention relates to a method of using a superfine pulverizing technology to prepare sterile powder for injection (powder injection) of chemical medicine and the prepared medicine powder injection. Invert sugar, clindamycin phosphate, cefpiramide sodium, cefepime hydrochloride, latamoxef sodium or cefmetazole sodium are preferable as the chemical medicine.
Owner:HAINAN LINGKANG PHARMA CO LTD
Cefmetazole sodium medicament and preparation method thereof
ActiveCN101623285AReduce contentUniform colorAntibacterial agentsOrganic active ingredientsSolubilityMedicine
The invention relates to a cefmetazole sodium medicament and a preparation method thereof. The cefmetazole sodium medicament contains 100 percent of cefmetazole sodium which is preprocessed by sterile refining. Because the cefmetazole sodium is preprocessed by sterile refining, the powder fluidity of the cefmetazole sodium medicament is improved to be beneficial to separated packing, reduce the packing difference caused in the separated packing process and have simpler process and easy operation. The cefmetazole sodium sterile powder for injection prepared by the method has uniform color, high purity, almost no impurity, reduced stimulation, good solubility, faster redissolution, better clarity after redissolution and more stable quality and can be stored for a long time.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +1
High-purified cefmetazole sodium compound
The invention provides a cefmetazole sodium compound, which is highly purified and finally obtained by the way of achieving the purification purpose through acid-base reaction, macroporous absorption resin and activated carbon adsorption, thus optimizing the quality of preparation products and the safety of clinical medication.
Owner:HAINAN LINGKANG PHARMA CO LTD
Method for preparing cefmetazole sodium
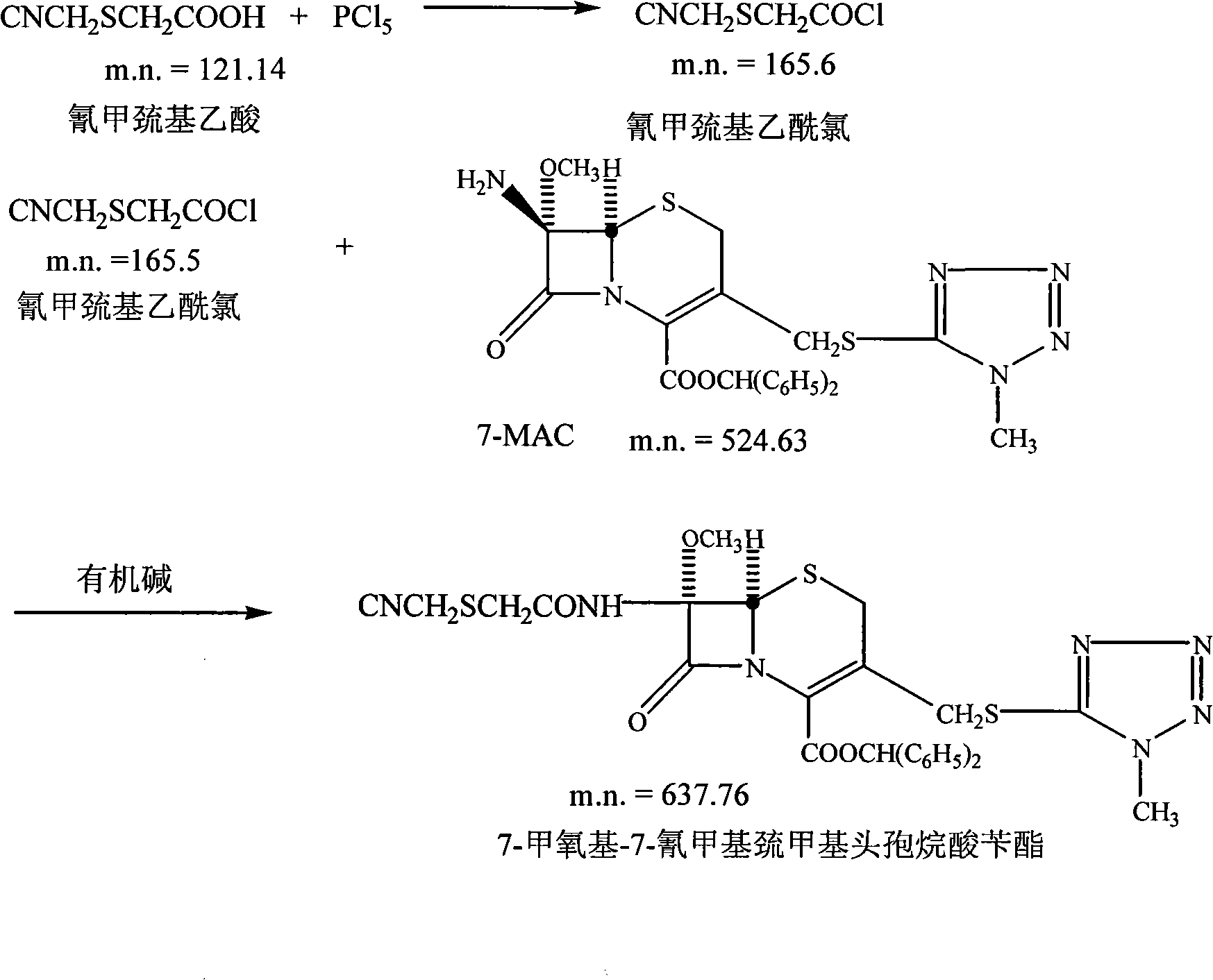
The invention relates to a method for preparing cefmetazole sodium, which comprises the following steps: (1) dissolving sodium hydroxide in water, adding mercaptoacetic acid and chloroacetonitrile into the mixture to perform the reaction, then adding sodium chloride and ethyl acetate into the reaction solution, stirring and dissolving the mixture, standing to separate phases and reserving a solvent phase for later use; (2) dissolving 7-MAC in methylene dichloride, adding organic base and side-chain solution into the mixture, stirring the mixed solution to perform the reaction to carry out phase separation, removing a water phase and carrying out secondary phase separation on the solvent phase to obtain cefmetazole benzyl ester; (3) dissolving ferric trichloride in aether, adding methylene dichloride in the mixture, dropwise adding the obtained product into cefmetazole acid to perform the reaction for 0.5 to 1 hour, then carrying out phase separation, removing the water phase, carrying out back extraction of alkali liquor on the solvent phase, then reserving the water phase, adding seed crystals, stirring the product and carrying out crystallization to obtain cefmetazole acid; and (4) dissolving the cefmetazole acid in alkali water and freezing out the mixture to obtain cefmetazole sodium. The preparation method of the invention has the advantages of 58 to 62 percent of yield, low cost, environment-friendly property, 92 percent of product purity, suitability for industrial production and the like.
Owner:哈药集团股份有限公司 +1
Cefmetazole sodium compound and synthetic method thereof
InactiveCN101550151AFew reaction stepsHigh yieldAntibacterial agentsOrganic chemistryAcetic acidCarboxylic salt
The invention relates to a cefmetazole sodium compound and a synthetic method thereof, 7 Beta-amino-7 Alpha-methoxyl-3-(1-methyl-1H-tetrazole-5-sulfidomethyl)-3-cephem-4-benzyl carboxylate and cyanomethylthio acetic acid sodium are mixed and react in the presence of p-toluenesulfonyl chloride to generate cefmetazole; sodium hydroxide is added to obtain cefmetazole sodium. Compared with the prior art, the invention has the advantages of few reaction steps, high productive rate, high product purity and low cost of raw materials, and has a broad prospect.
Owner:HAINAN LINGKANG PHARMA CO LTD
Method for preparing cefmetazole sodium
InactiveCN102127095AThe preparation method is scientific and reasonableHigh product contentOrganic chemistryQuality performanceCEFMETAZOLE SODIUM
The invention relates to a method for preparing cefmetazole sodium, comprising the following steps of: undergoing a reaction of cefmetazole acid and sodium isocaprylate, then purifying, crystallizing, filtering, washing and drying to obtain a finished product. The method for preparing the cefmetazole sodium, provided by the invention, is scientific and reasonable, simple and practicable; and the obtained product has the advantages of stable quality performance, high content, low impurity content, allergy avoidance, and the like.
Owner:SHANDONG LUKANG PHARMA
Cefmetazole aseptic powder and its preparation method
ActiveCN102627660AHigh puritySolve the problem of easily oxidized impuritiesOrganic active ingredientsAntimycoticsActivated carbonSolvent
The invention relates to the field of pharmaceutics, specifically to cefmetazole aseptic powder and its preparation method. The preparation method comprises: employing a mixed solvent to recrystallize cefmetazole, using a good solvent to dissolve the cefmetazole raw material, adding activated carbon for injection to remove a pyrogen, then adding a poor solvent for recrystallization, and conducting drying at a low temperature, thus obtaining the cefmetazole aseptic powder with no pyrogen and high purity. Characterized by simple operation and high yield, the preparation method of the invention can prepare cefmetazole aseptic powder with high purity, safe and reliable quality, thus being suitable for preparing cefmetazole aseptic powder injections and widely applicable in large scale production of cefmetazole aseptic powder injections.
Owner:HAINAN JINXING PHARMA
Cefmetazole sodium composition powder injection for injection
ActiveCN101862296ALarge master granularityLarge granularityAntibacterial agentsOrganic active ingredientsGranularitySodium benzoate
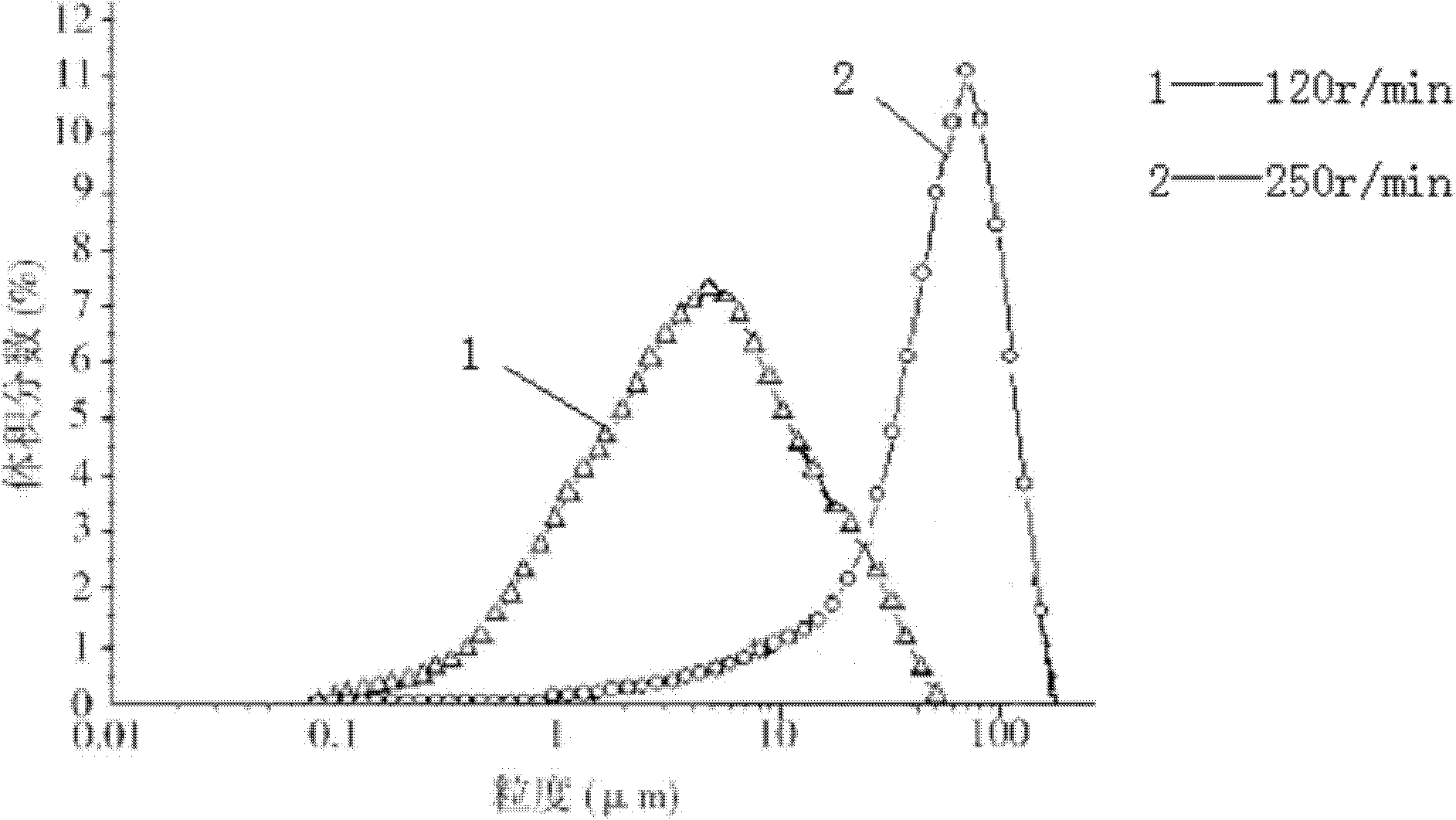
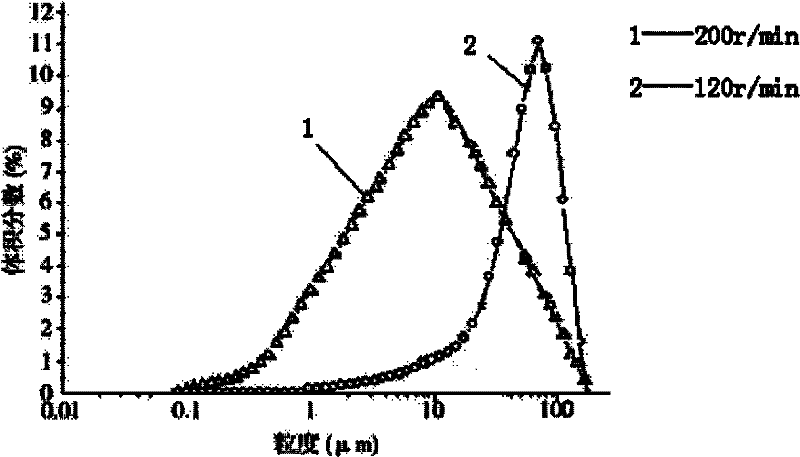
The invention relates to cefmetazole sodium composition sterile powder for injection, which comprises 99.0 to 99.9 weight percent of cefmetazole sodium and 0.1 to 1.0 percent of sodium benzoate. The cefmetazole sodium is cefmetazole sodium crystal and the primary granularity of the cefmetazole sodium ranges from 80 to 120 mu m. The cefmetazole sodium composition sterile powder for injection provided by the invention contains the cefmetazole sodium crystal, and has the advantages of large crystal granularity, uniform granularity distribution, qualified quality, and high stability.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Pharmaceutical composition containing cefmetazole sodium compound, and preparation method thereof
ActiveCN102204916AQuality improvementFor long-term storageAntibacterial agentsOrganic active ingredientsActive componentBULK ACTIVE INGREDIENT
The invention provides a pharmaceutical composition containing a cefmetazole sodium compound. The pharmaceutical composition is prepared from cefmetazole acid, sodium carbonate and mannitol. The invention also provides a preparation method of the pharmaceutical composition containing the cefmetazole sodium compound. The preparation method comprises the following steps of: adding the active component cefmetazole acid sterile powder, the cosolvent sodium carbonate and the excipient mannitol to water for injection, adding active carbon and stirring, regulating the pH to be 6 by sodium hydroxide, filtering to remove the active carbon, finely filtering by a filter membrane, filling and lyophilizing. Because a proper amount of excipient mannitol is added to the pharmaceutical composition prepared by the method, the lyophilized powder is more stable in quality and can be stored for a long term. The cefmetazole acid and the sodium carbonate directly react to obtain the cefmetazole sodium, thereby shortening the process flow.
Owner:福建亿懿兴华生物技术开发有限公司
Special ultrafine cefmetazole sodium powder preparation and preparation method thereof
InactiveCN104163822AHigh clarityImprove stabilityAntibacterial agentsOrganic active ingredientsSodium bicarbonateEthyl acetate
The invention discloses a special ultrafine cefmetazole sodium powder preparation and a preparation method thereof. The preparation method comprises the following steps: carrying out a reaction on a raw material cefmetazole benzyl ester in a dichloromethane, anisole and iron trichloride system, carrying out acidolysis by using a hydrochloric acid and acetone mixed solution, purifying the above collected organic phase by using a sodium chloride solution, carrying out phase inversion by using a sodium bicarbonate solution, decoloring the above collected water phase by active carbon and alumina, carrying out phase inversion by ethyl acetate and hydrochloric acid, crystallizing by adding crystal seeds in order to prepare cefmetazole acid, reacting cefmetazole acid with sodium bicarbonate to prepare cefmetazole sodium, purifying, and carrying out air jet ultrafine crushing to prepare the special ultrafine cefmetazole sodium powder preparation. The special ultrafine cefmetazole sodium powder preparation has the advantages of good clarity, high stability, high purity, few impurities, small particle size, large specific surface area, good solubility, small toxic side effects, difficult allergy and the like.
Owner:杭州长典老一元健康管理有限公司
Cefmetazole-containing pharmaceutical composition
ActiveCN102727451ASmall fluctuation rangeGuaranteed stabilityAntibacterial agentsOrganic active ingredientsHydrogenVitamin C
The invention relates to a novel pharmaceutic preparation composition, especially to an injection preparation of cefmetazole. The injection preparation comprises the following components: cefmetazole, mannitol, EDTA-Ca, vitamin C, sodium hydrogen citrate, a trisodium citrate buffer solution and water for injection.
Owner:哈药集团股份有限公司 +1
Composition of cefmetazole acid
InactiveCN101548977AEasy to purifyImprove product qualityPowder deliveryOrganic active ingredientsSide effectArginine
The invention relates to a composition of a cefmetazole acid, which is characterized in that the mix of cefmetazole acid and arginine aseptic powders with the weight ratio of 1: 0.35 to 1:0.70 is dissolved in water. The invention includes the detail steps of weighting 50kg material drug of cefmetazole acid and arginine aseptic powders by the weight percentage of 1: 0.35 to 1:0.70; pouring the material drug in a three-dimensional motion mixer and setting the rotating speed of the three-dimensional motion into 5 rotation per minute; well mixing the material drug for 60 minutes to 90 minutes; discharging the material and sub-packaging the material. The invention has the advantages that the composition improves the quality of products and reduces impurities, the composition markedly improves the stability of product, effectively reduces the content of impurities and solves the problem of water insolvability, and cefmetazole acid is more stable than cefmetazole sodium and can be purified easily; if cefmetazole acid is used to obtain preparation together with arginine proportionally, the all relative material can be stabilized in 2 percent for a long time, thereby reducing the incidence rate of side effects and allergy and achieving actual meaning and value.
Owner:国药集团致君(苏州)制药有限公司
Cefmetazole sodium liposome freeze-dried preparation and preparation method
InactiveCN102106830AImprove stabilityAntibacterial agentsOrganic active ingredientsFreeze-dryingPharmaceutical drug
The invention provides a prescription of a cefmetazole sodium liposome freeze-dried preparation and a preparation method. The method comprises the following steps of: adding cefmetazole sodium, a stabilizing agent and an excipient into a blank membrane material made of a liposome carrier, performing ultrasonic processing, fixing volume, filling, and freeze drying to obtain the cefmetazole sodium liposome freeze-dried preparation. The cefmetazole sodium liposome freeze-dried preparation prepared by the method overcomes the defect of formulations appear on the market, has improved stability and is safer in clinical application of medicaments.
Owner:张宏民
Cephamycin intermediate compound and preparation method thereof
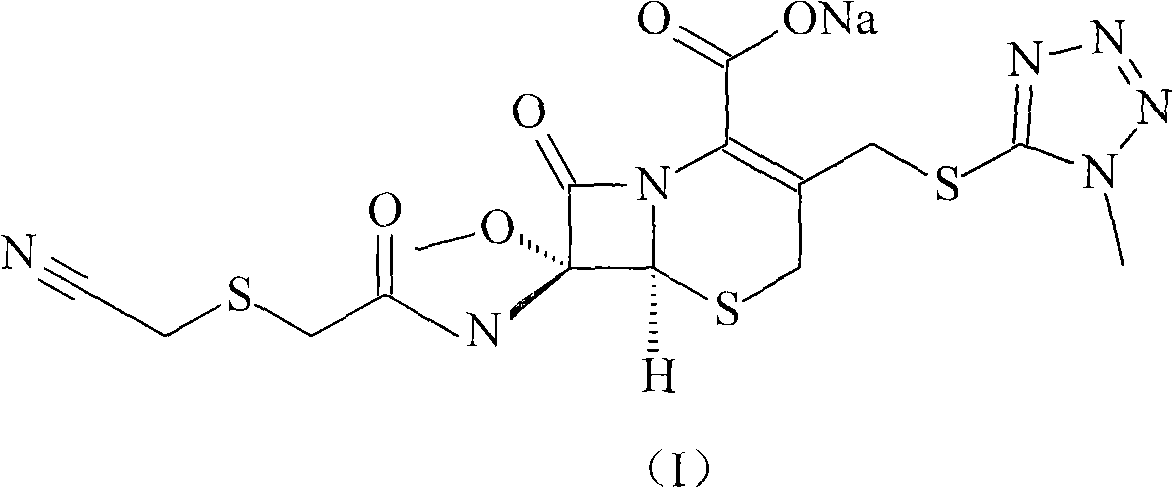
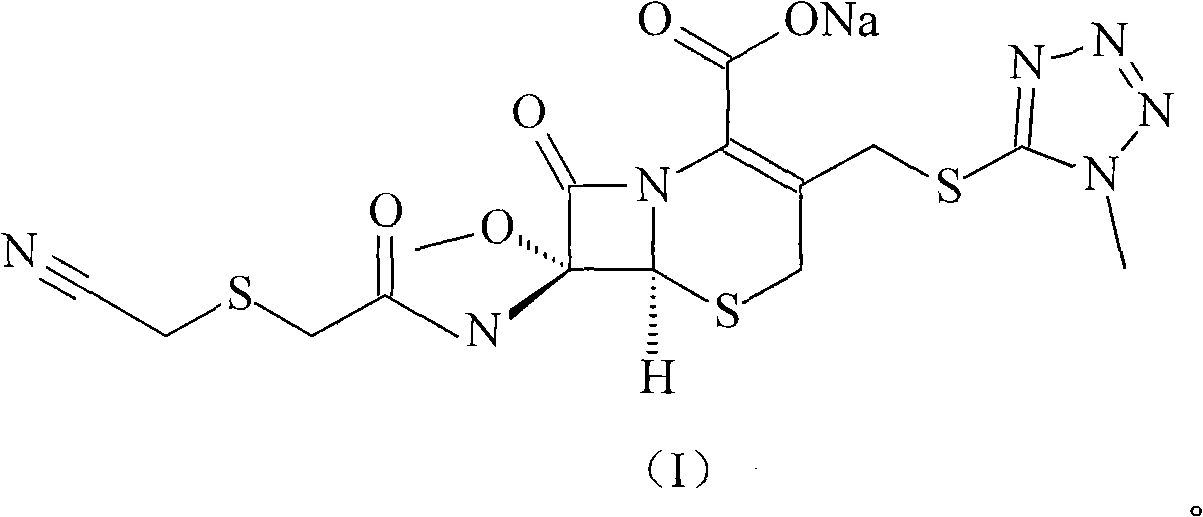
ActiveCN103193796ARaise the level of purityHigh purityOrganic compound preparationAmino compound preparationCEFMINOX SODIUMCarboxylic acid
The invention relates to a cephamycin intermediate compound and a preparation method thereof. The cephamycin intermediate compound has a structure of formula (I), and is prepared by directly reacting 7beta-chloroacetamide-7alpha-methoxy-3-(1-methyl-1H-tetrazole-5-thiomethyl)-3-cephem-4-carboxylic acid and dicyclohexylamine in a specific solvent to form a salt and crystallizing. The cephamycin intermediate compound has the characteristics of high purity, high stability and the like, is conductive to improving the purity of cephamycin final products such as cefminox sodium, cefmetazole sodium and cefotetan disodium, and is beneficial to large-scale production and application.
Owner:山东安弘制药有限公司
Injection-use pharmaceutical composition comprising cefmetazole sodium and clavulanate potassium
ActiveCN103271925AEnhanced inhibitory effectGood resolubilityAntibacterial agentsOrganic active ingredientsGlycineMANNITOL/SORBITOL
The invention provides an injection-use pharmaceutical composition comprising cefmetazole sodium and clavulanate potassium, and the pharmaceutical composition has a cooperatively antibacterial effect. The pharmaceutical composition comprises the following ingredients: in parts by weight, 1-4 parts of the cefmetazole sodium; 1-2 parts of the clavulanate potassium; 2-6 parts of polyalkylcyanoacrylate; 0.2-0.5 parts of L-arginine; 0.5-1 parts of PVP; 0.5-1 parts of mannitol; 0.5-1 parts of glycine; 1-2 parts of a tween. The pharmaceutical composition has the advantages of high stability, good redissolubility, simple preparation processes, a good inhibitory effect on ESBLs-producing escherichiacoli, klebsiellapeumoniae, and proteusmirabilis, and a definite curative effect, can reduce the dosage of a single prescription preparation and shorten the treatment cycle, and has broad market prospects.
Owner:FUAN PHARM (GRP) CO LTD +1
New cefmetazole sodium for injection
InactiveCN108014091ASimple preparation processLow costAntibacterial agentsOrganic active ingredientsBlood concentrationLactide
The invention relates to new cefmetazole sodium for injection and a preparation method thereof, and belongs to the technical field of medicines. The new cefmetazole sodium for injection is prepared from 1 part of cefmetazole sodium, 1.13 to 1.18 parts of gelatin, 1.36 to 1.38 parts of arabic gum, 0.4 to 0.5 part of plasticizer, 0.8 to 1.2 parts of stabilizing agent and 0.9 to 1 part of anti-sticking agent. The stabilizing agent dioxanone-lactide copolymer is a copolymer in a special proportion and plays an important function on stability of a preparation. According to an injection, through a slow release effect of a microcapsule, a medicine application method of 2 to 4 times everyday is changed into medicine application for one time, so that medicine application intervals are shortened, the new cefmetazole sodium is convenient to use, the bioavailability is improved, the medicine using amount is reduced, so that the blood concentration is stable to improve a curative effect, and an adverse reaction is reduced. The preparation process of the cefmetazole sodium for injection provided by the invention is simple, is low-cost, and is suitable for large scale production; therefore, the new cefmetazole sodium is a product having market prospect.
Owner:石药集团中诺药业(石家庄)有限公司
Cefmetazole-containing and beta-lactamase inhibitor-containing medicinal composition
ActiveCN101816791AAntibacterial agentsHeterocyclic compound active ingredientsCEFMETAZOLE SODIUMDrug tolerance
The invention provides a cefmetazole-containing and beta-lactanase inhibitor-containing medicinal composition, belongs to the technical field of medicaments, in particular relates to a medicinal composition consisting of 1 to 8 weight parts of cefmetazole and 1 weight part of specific beta-lactamase inhibitor. The beta-lactamase inhibitor is one of sulbactam sodium and tazobactam sodium. The medicinal composition can improve the antimicrobial spectrum of the medicament cefmetazole sodium, and reduce the drug tolerance of bacteria.
Owner:HAINAN TIANHUANG PHARMA +2
Preparation method of cefmetazole sodium
InactiveCN102718780ADifficulty avoiding crystallizationResidue reductionOrganic chemistryFreeze-dryingFiltration
The invention discloses a preparation method of cefmetazole sodium, comprising the following steps: (1) dissolving phosphorus pentachloride in dichloromethane, then adding cyanomethylthio potassium acetate to prepare a side-chain acyl chloride solution; dissolving 7-MAC in dichloromethane, and adding organic base and the above side-chain acyl chloride solution to wash to obtain a cefmetazole benzyl ester solution; (2) adding the cefmetazole benzyl ester solution in an aluminum trichloride anisole solution, washing, regulating the pH value to 6.0-9.0, crystallizing, filtering and drying to obtain cefmetazole amine salt; and (3) dissolving the cefmetazole amine salt in an organic solvent solution, adding cation exchange resin, removing the cation exchange resin by filtration, regulating the pH to 5-8, then removing the organic solvent by distillation at reduced pressure, letting the residual cefmetazole sodium solution be subject to decoloration and aseptic filtration, and then directly conducting freeze-drying to obtain cefmetazole sodium. The method is simple and practicable and has the advantages of high yield, good quality and low cost.
Owner:刘伟娜
Preparation method for cefmetazole sodium
The invention discloses a preparation method for cefmetazole sodium. The preparation method for the cefmetazole sodium comprises the following operation steps: (1), adding chloroform to 7-MAC as an initial raw material, stirring and dissolving; adding alkali, dropping cyanomethylthioacetyl chloride, and stirring to react to obtain cefmetazole diphenylmethyl ester; (2), enabling the cefmetazole diphenylmethyl ester obtained in the step (1) to react in an alchlor-anisole system to obtain an organic solution of cefmetazole acid; (3), preparing a sodium bicarbonate solution with the concentration of 5-20 wt%, controlling the temperature within a range from 5 DEG C below zero to 10 DEG C, adding the organic solution of cefmetazole acid, which is obtained in the step (2), stirring, leaving a mixture to stand and layer, taking out a water layer, adding activated carbon, stirring to decolorize, controlling the temperature to 0-5 DEG C, adding neutral alumina, stirring to decolorize, filtering to obtain a filtrate, and freeze-drying the filtrate to obtain the cefmetazole sodium. The preparation method for the cefmetazole sodium has the advantages of high yield, excellent product quality and convenience in industrial production.
Owner:福安药业集团重庆博圣制药有限公司 +1
Novel cefmetazole compound and medicine composition thereof
ActiveCN103044459ANot prone to oxidative decompositionImprove stabilityAntibacterial agentsOrganic active ingredientsMANNITOL/SORBITOLSide effect
The invention discloses a novel cefmetazole compound, which has the purity greater than 99.5%. The invention further discloses a medicine composition of the cefmetazole compound. The medicine composition comprises the following components in part by weight: 60 to 90 parts of cefmetazole, 0.5 to 2 parts of mannitol, 2 to 7 parts of citrulline, and 1 to 5 parts of sodium citrate. The mannitol, the citrulline, and the sodium citrate contained in the medicine composition can work together to greatly improve light stability and heat stability of cefmetazole medicine composition, the cefmetazole medicine composition is less susceptible to oxygenolysis, and the probability of side effect of cefmetazole is effectively reduced.
Owner:石药集团中诺药业(石家庄)有限公司
Cefmetazole sodium suspension injection powder and novel application thereof
InactiveCN101780053AIncrease spawn rateEasy to wrapPowder deliveryOrganic active ingredientsEmulsionWhole body
The invention provides cefmetazole sodium suspension injection powder and a preparation method thereof. The cefmetazole sodium suspension injection powder comprises the following components by weight part: 10 parts of cefmetazole sodium, 5 to 30 parts of biodegradable polymer receivable on pharmacy, 1 to 20 parts of emulsifier, 6 to 12 parts of emulsion assistant and 2 to 30 parts of skeleton agent. The cefmetazole sodium suspension injection powder has the application for treating body extensive Psoriasis Pustulosa.
Owner:HAINAN LINGKANG PHARMA CO LTD
Cefmetazole sodium liposome freeze-dried preparation and preparation method
The invention provides a cefmetazole sodium liposome freeze-dried preparation and a preparation method. The method comprises the following steps of: adding cefmetazole sodium, a stabilizing agent and an excipient into a blank membrane material made of a liposome carrier, performing ultrasonic processing, fixing volume, filling, and freeze drying to obtain the cefmetazole sodium liposome freeze-dried preparation. The cefmetazole sodium liposome freeze-dried preparation prepared by the method overcomes the defect of formulations appear on the market, has improved stability and is safer in clinical application of medicaments.
Owner:张宏民
Preparation method for cephamycin intermediate
The invention relates to a preparation method for a cephamycin intermediate. A compound of the cephamycin intermediate can be seen in the formula (IV). The cephamycin intermediate is obtained by making 7-ACA react with dichloroacetyl chloride in specific solvent, making the obtained product react with MMT under catalysis of a sulfoacid catalyst and conducting crystallization. The cephamycin intermediate has the advantages of being high in purity and stability and the like, is beneficial for improving the purity of final cephamycin products such as cefminox sodium, cefmetazole and cefotetan disodium, and is beneficial to large-scale production and application.
Owner:山东安弘制药有限公司
Cefmetazole sodium compound and preparation method thereof
InactiveCN104844627AReduce usageImprove securityAntibacterial agentsOrganic active ingredientsCombinatorial chemistryKetone
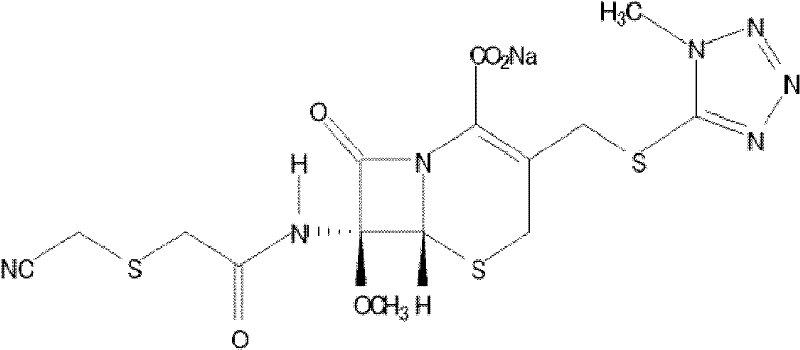
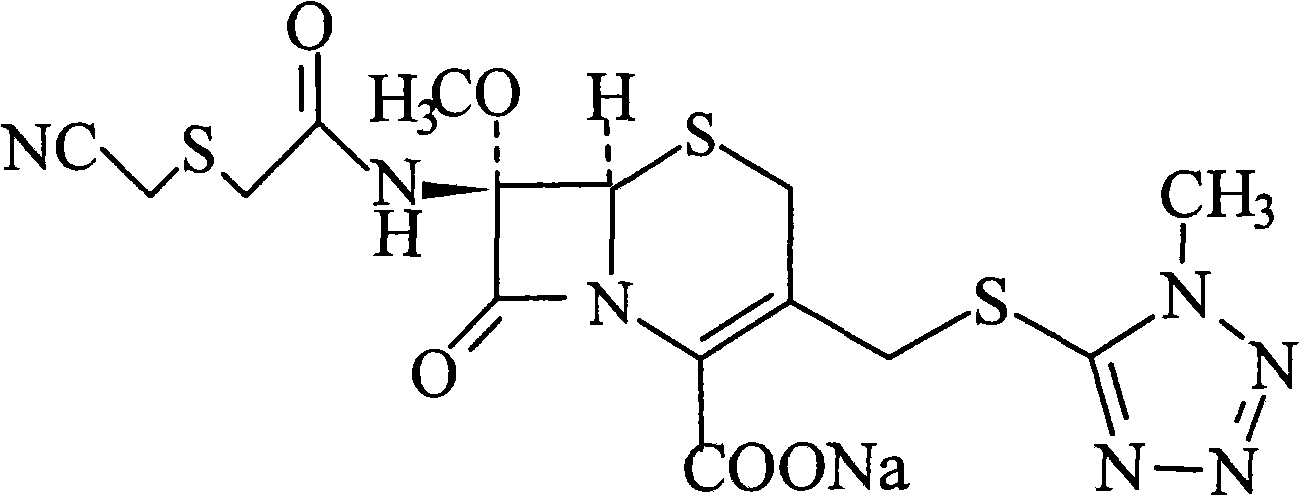
The invention relates to the field of pharmaceutical chemistry, and concretely provides a new cefmetazole sodium crystal form compound. The crystal form compound is obviously different from other cefmetazole sodium crystal forms in X-ray powder diffraction pattern characteristics, the purity of the new cefmetazole sodium crystal form compound is above 99.5%, and the new cefmetazole sodium crystal form compound also has the advantages of low content of impurities, uniform particle size distribution and good stability. The invention also provides a preparation method of the crystal form compound, and an application of the crystal form compound in a medicinal composition. The preparation method comprises the following steps: dissolving cefmetazole sodium in an aprotic polar good solvent, sequentially adding an alcoholic anti-solvent and an ether and ketone mixture in a dropwise manner, carrying out room temperature stirring, cooling for crystallization, filtering, and drying to prepare cefmetazole sodium crystals. The preparation method has the advantages of simplicity, easily available device, and suitableness of industrial mass production.
Owner:YAOPHARMA CO LTD +1
Cefmetazole sodium powder for injection and preparation method thereof
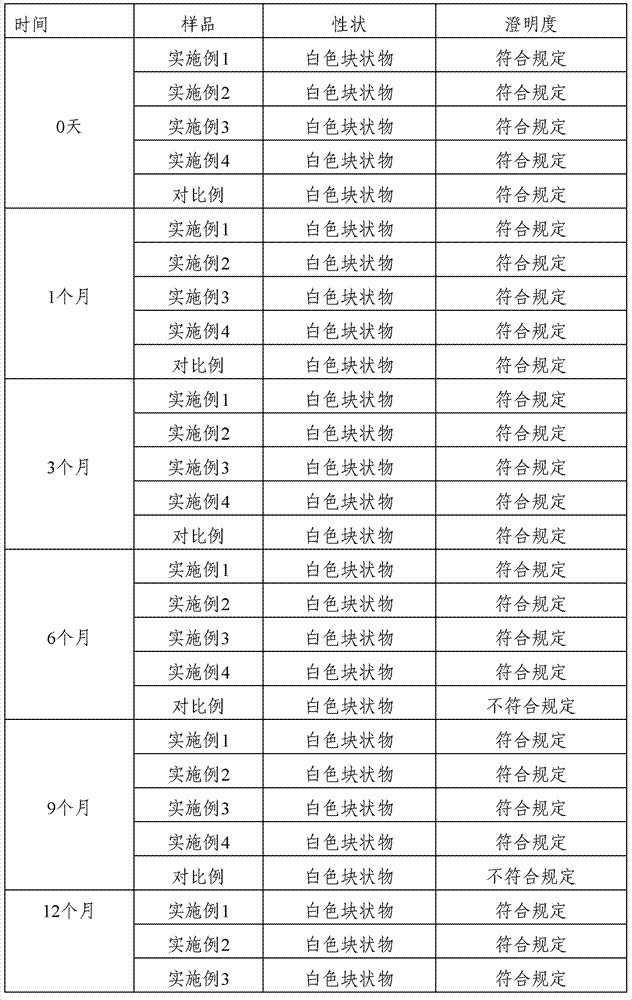
ActiveCN102824309AReasonable compositionSimple preparation processAntibacterial agentsOrganic active ingredientsBottleTubing types
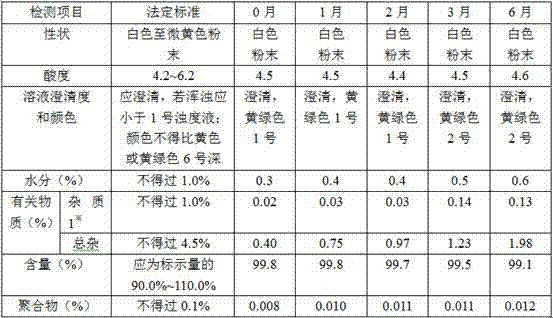
The invention discloses a cefmetazole sodium powder for injection. The cefmetazole sodium powder for injection comprises: by weight, 1000 parts of cefmetazole sodium used as a raw material and 10-20 parts of L-lysine used as an auxiliary material. The power injection is prepared according to the following preparation method: firstly removing dust from the raw material and the auxiliary material, cleaning, wiping and sterilizing; respectively crushing cefmetazole sodium and L-lysine in a clean area of 100 level, and sieving through a sieve of 80 meshes; weighing and mixing according to a prescription, subpackaging into tube-type bottles after testing content qualified, pressing plugs, capping, carrying out lamp inspection, examining qualified, labeling and packaging. The cefmetazole sodiumpowder for injection in the prescription has stable performance to temperature and light. After 6 months of accelerated testing and 12 months of long-term sample storage for observation, contents of related substances are all less than 1%, and color, clarity, bacterial endotoxin and the like of the powder injection all accord with the pharmacopeial standard. The preparation method of the prescription has advantages of simple technology and stable and reliable quality.
Owner:YOUCARE PHARMA GROUP
Cefmetazole sodium composition powder injection for injection
ActiveCN101862296BLarge master granularityLarge granularityAntibacterial agentsOrganic active ingredientsGranularitySodium benzoate
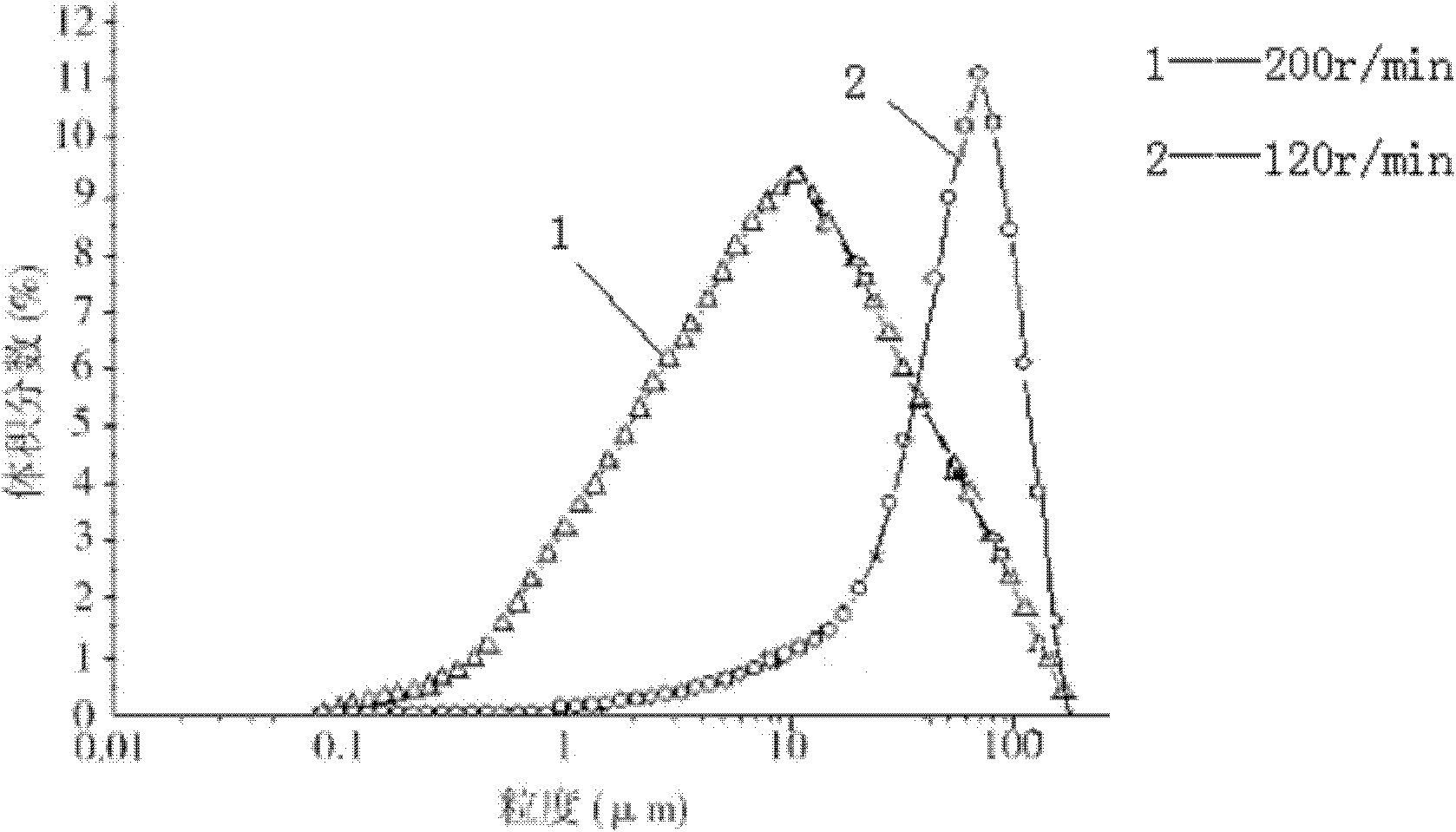
The invention relates to cefmetazole sodium composition sterile powder for injection, which comprises 99.0 to 99.9 weight percent of cefmetazole sodium and 0.1 to 1.0 percent of sodium benzoate. The cefmetazole sodium is cefmetazole sodium crystal and the primary granularity of the cefmetazole sodium ranges from 80 to 120 mu m. The cefmetazole sodium composition sterile powder for injection provided by the invention contains the cefmetazole sodium crystal, and has the advantages of large crystal granularity, uniform granularity distribution, qualified quality, and high stability.
Owner:SHANDONG LUOXIN PHARMA GRP CO LTD
A kind of cefmetazole sodium compound and pharmaceutical composition thereof
ActiveCN103044459BNot easy to oxidize and decomposeNot prone to oxidative decompositionAntibacterial agentsOrganic active ingredientsSide effectMedicine
Owner:石药集团中诺药业(石家庄)有限公司
Preparation method of cefmetazole
The invention relates to a preparation method of cefmetazole. The preparation method comprises the steps of adopting a one-pot method containing 7-ACA, 1-methyl-5-tetrazole-thione and chloroacetyl chloride, obtaining 7-DCT, then using seven-position methoxy groups of sodium methylate and tert-butyl hypochlorite, and obtaining methoxy cephalosporin mother nuclei which are shared mother nuclei. The cefmetazole can serve as four methoxy cephalosporin products, namely cefminox, cefmetazole, cephalosporin and cefotetan disodium, three steps are omitted, the processing steps are greatly shortened, cost is lowered, and the overall yield is raised to 70%.
Owner:NANJING JINHAO MEDICAL TECH CO LTD
A kind of cefmetazole sodium aseptic powder and preparation method thereof
ActiveCN102627660BHigh puritySolve the problem of easily oxidized impuritiesOrganic active ingredientsAntimycoticsActivated carbonSolvent
The invention relates to the field of pharmaceutics, specifically to cefmetazole aseptic powder and its preparation method. The preparation method comprises: employing a mixed solvent to recrystallize cefmetazole, using a good solvent to dissolve the cefmetazole raw material, adding activated carbon for injection to remove a pyrogen, then adding a poor solvent for recrystallization, and conducting drying at a low temperature, thus obtaining the cefmetazole aseptic powder with no pyrogen and high purity. Characterized by simple operation and high yield, the preparation method of the invention can prepare cefmetazole aseptic powder with high purity, safe and reliable quality, thus being suitable for preparing cefmetazole aseptic powder injections and widely applicable in large scale production of cefmetazole aseptic powder injections.
Owner:HAINAN JINXING PHARMA
Pharmaceutical composition containing cefmetazole sodium compound, and preparation method thereof
ActiveCN102204916BQuality improvementFor long-term storageAntibacterial agentsOrganic active ingredientsActive componentBULK ACTIVE INGREDIENT
The invention provides a pharmaceutical composition containing a cefmetazole sodium compound. The pharmaceutical composition is prepared from cefmetazole acid, sodium carbonate and mannitol. The invention also provides a preparation method of the pharmaceutical composition containing the cefmetazole sodium compound. The preparation method comprises the following steps of: adding the active component cefmetazole acid sterile powder, the cosolvent sodium carbonate and the excipient mannitol to water for injection, adding active carbon and stirring, regulating the pH to be 6 by sodium hydroxide,filtering to remove the active carbon, finely filtering by a filter membrane, filling and lyophilizing. Because a proper amount of excipient mannitol is added to the pharmaceutical composition prepared by the method, the lyophilized powder is more stable in quality and can be stored for a long term. The cefmetazole acid and the sodium carbonate directly react to obtain the cefmetazole sodium, thereby shortening the process flow.
Owner:福建亿懿兴华生物技术开发有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com