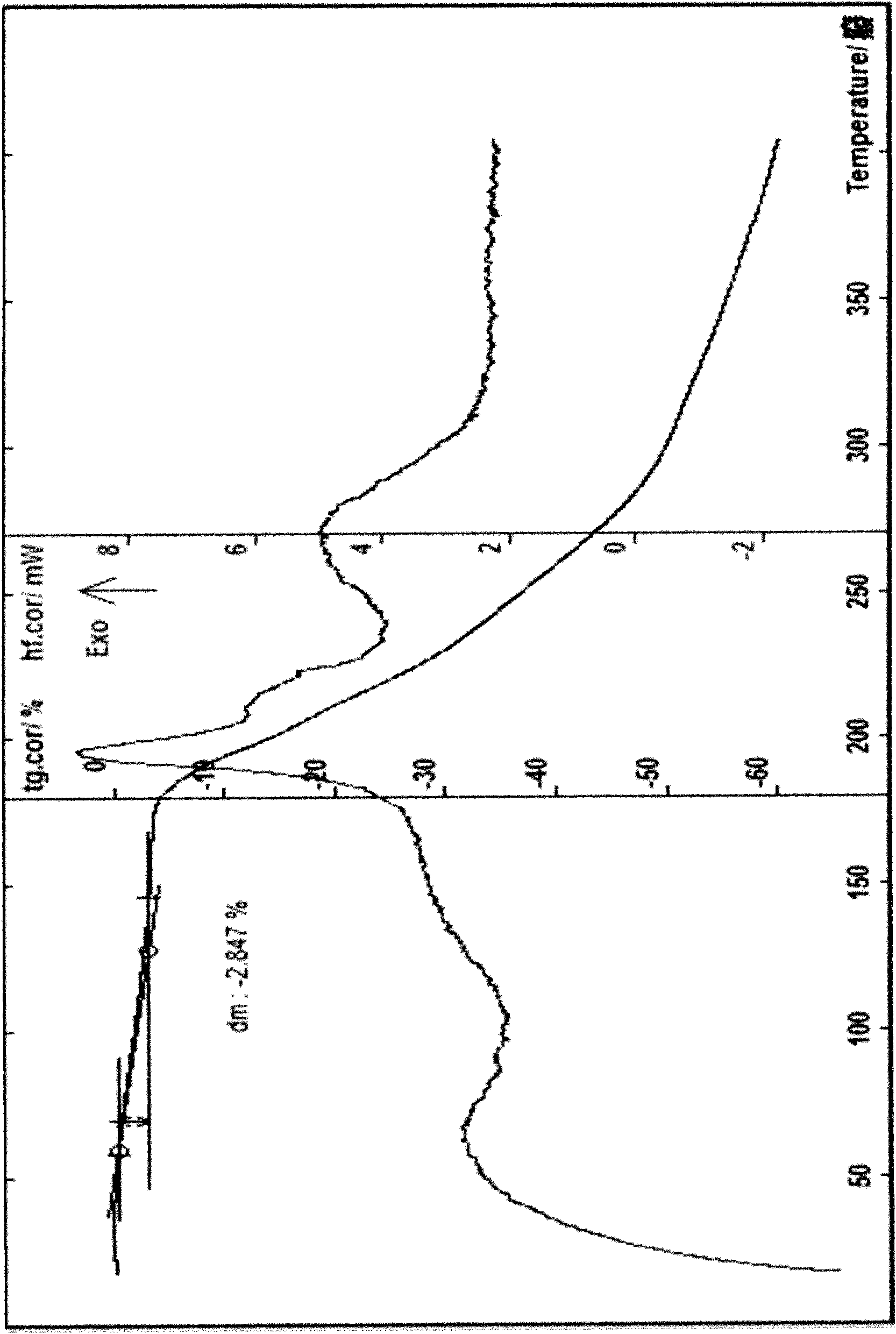
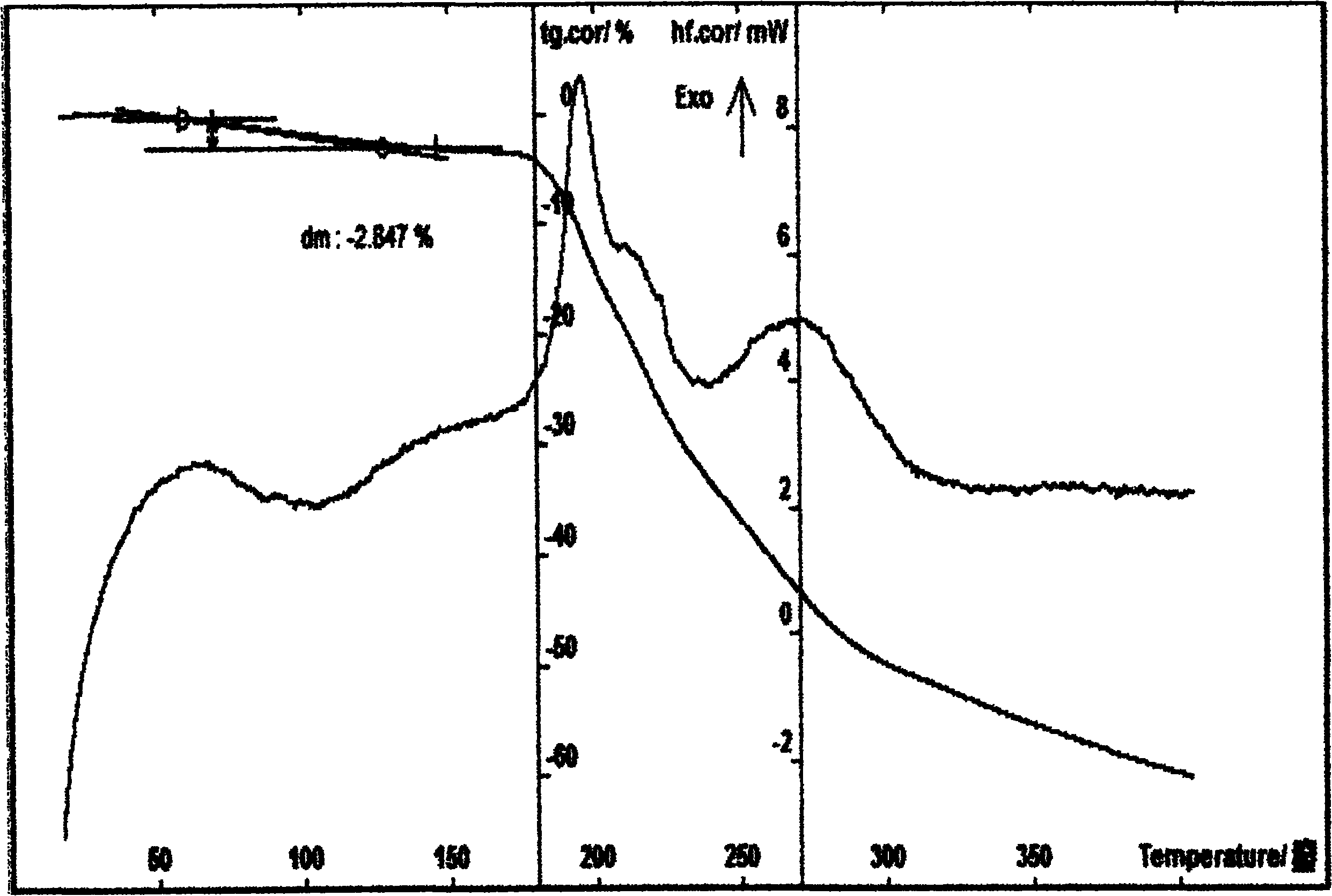
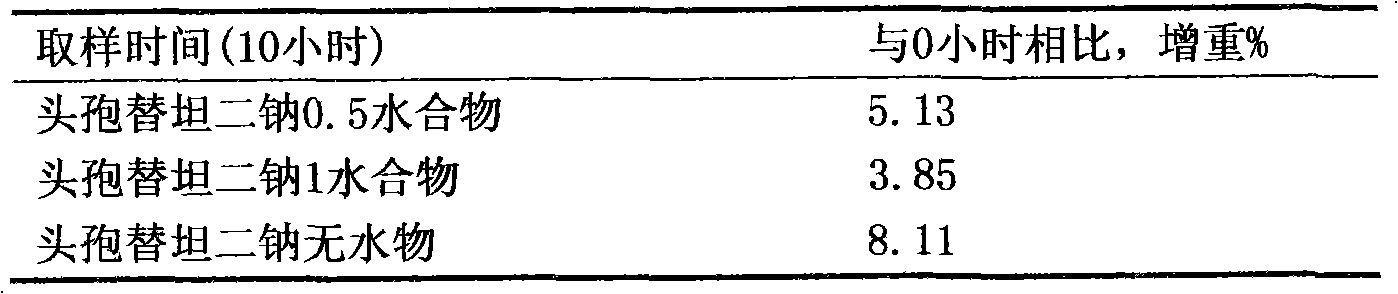
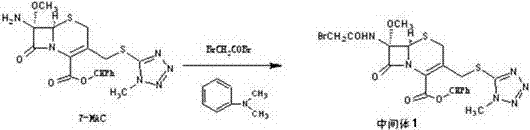
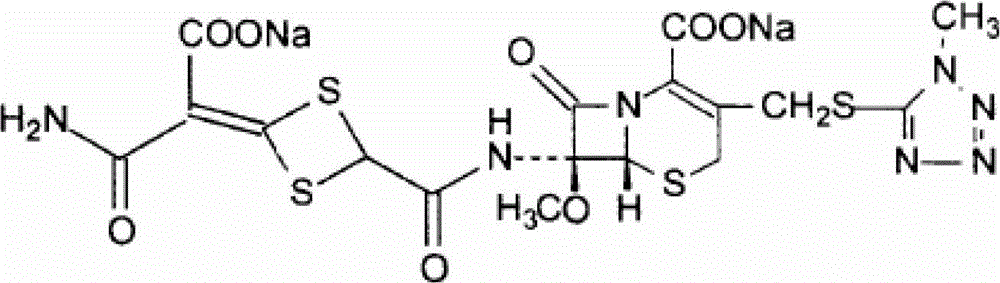
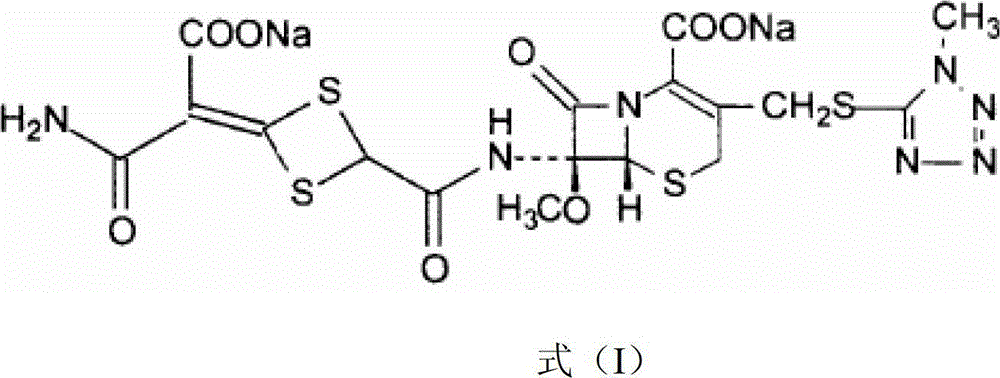
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
31 results about "Cefotetan Disodium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
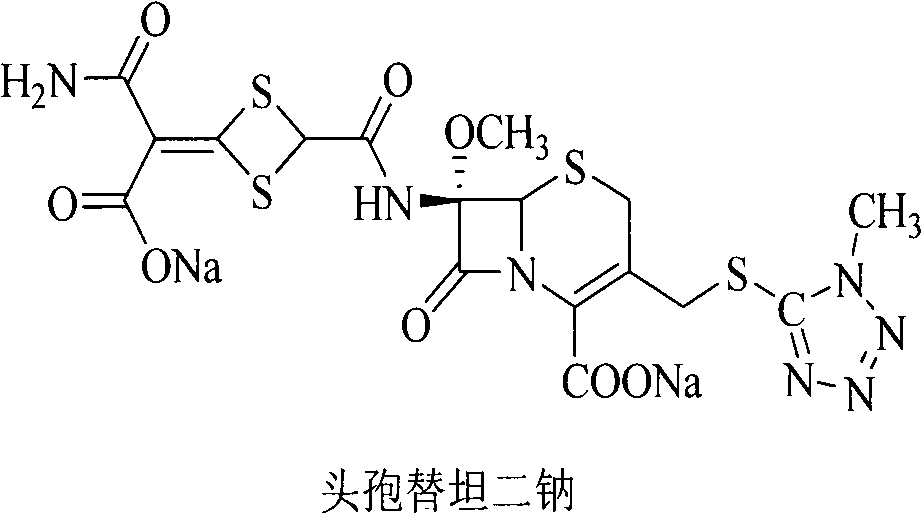
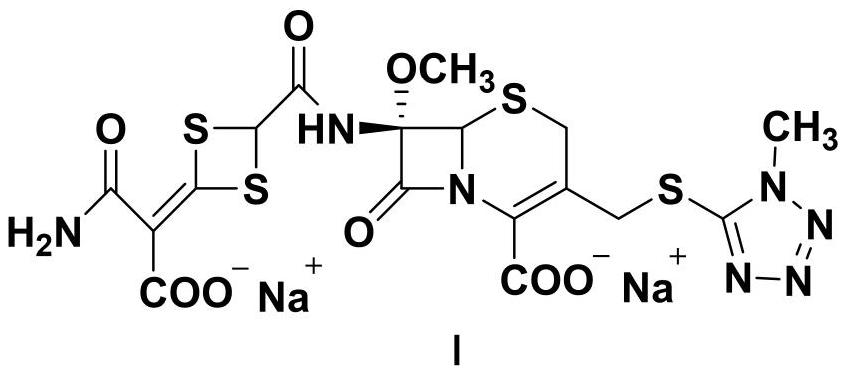
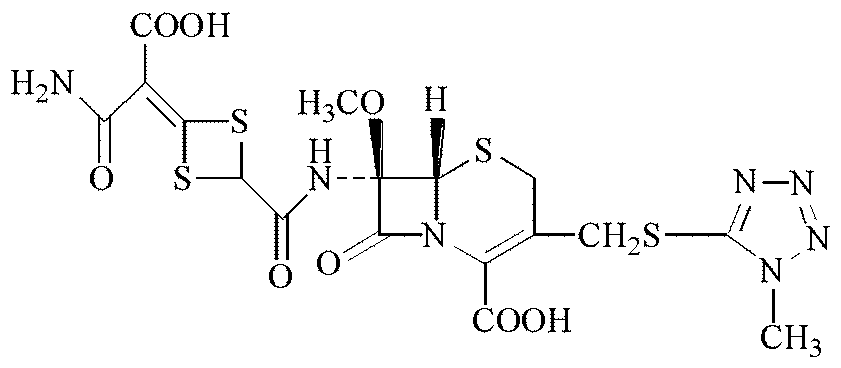
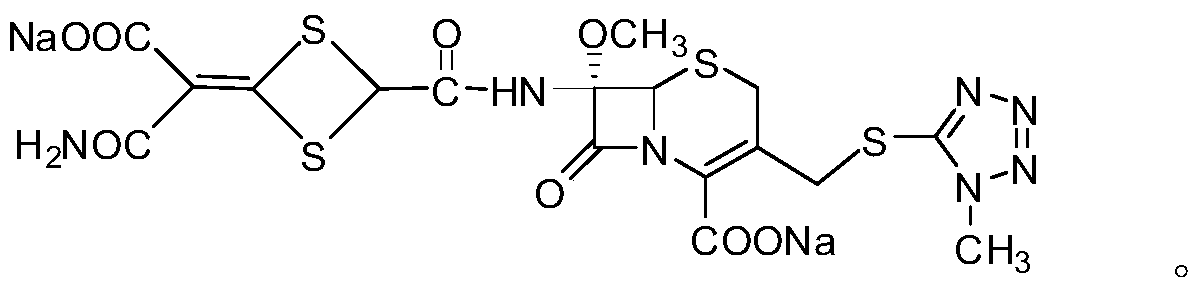
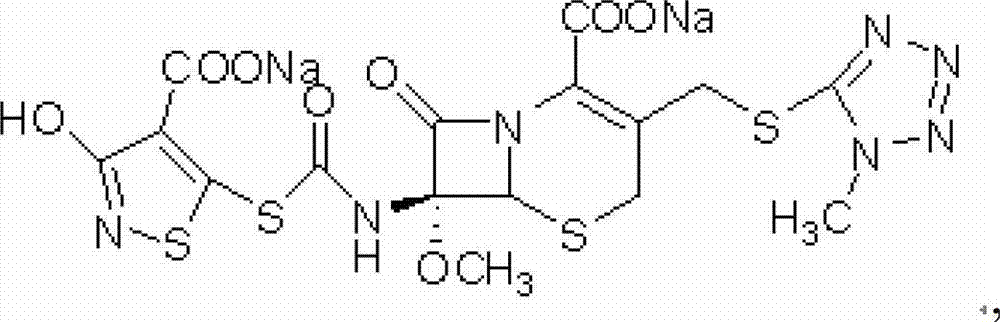
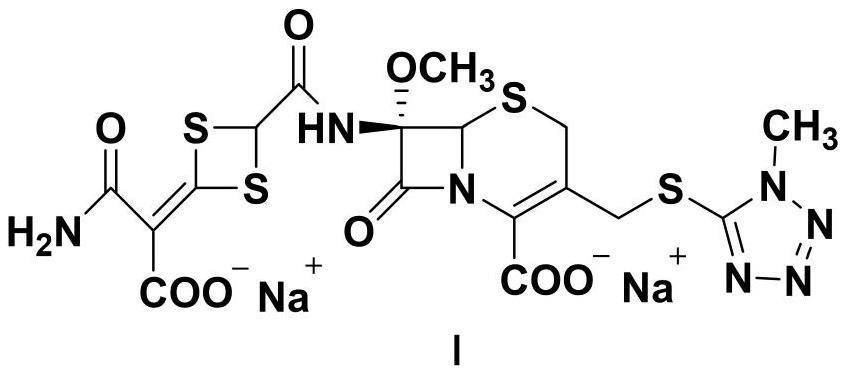
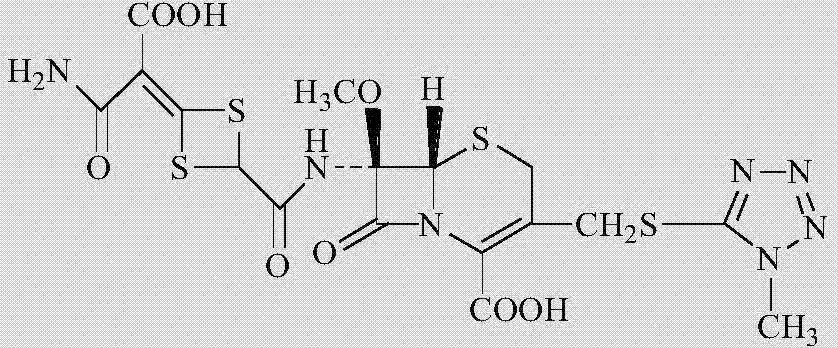
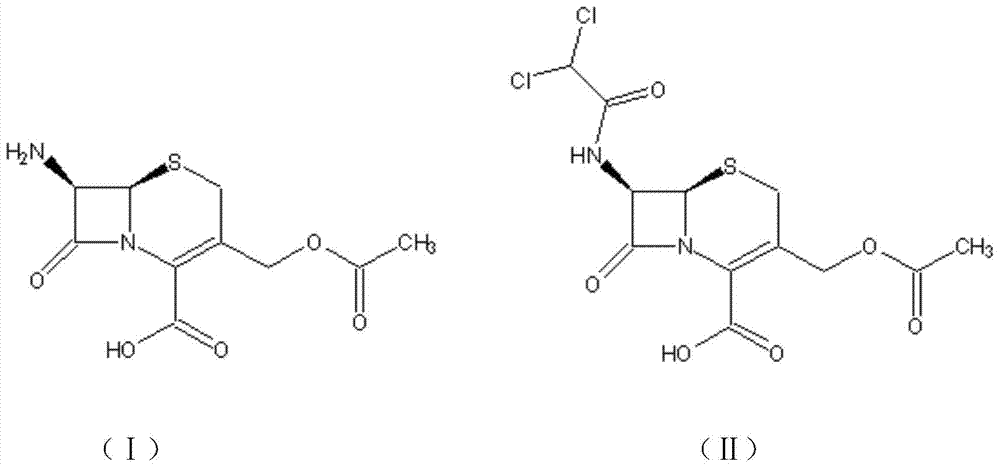
The disodium salt form of cefotetan and a semi-synthetic, broad-spectrum, beta-lactamase-resistant, second-generation cephalosporin antibiotic with bactericidal activity. Cefotetan disodium causes inhibition of bacterial cell wall synthesis by inactivating penicillin binding proteins (PBPs) thereby interfering with the final transpeptidation step required for cross-linking of peptidoglycan units which are a component of the cell wall. This results in a reduction of cell wall stability and causes cell lysis.
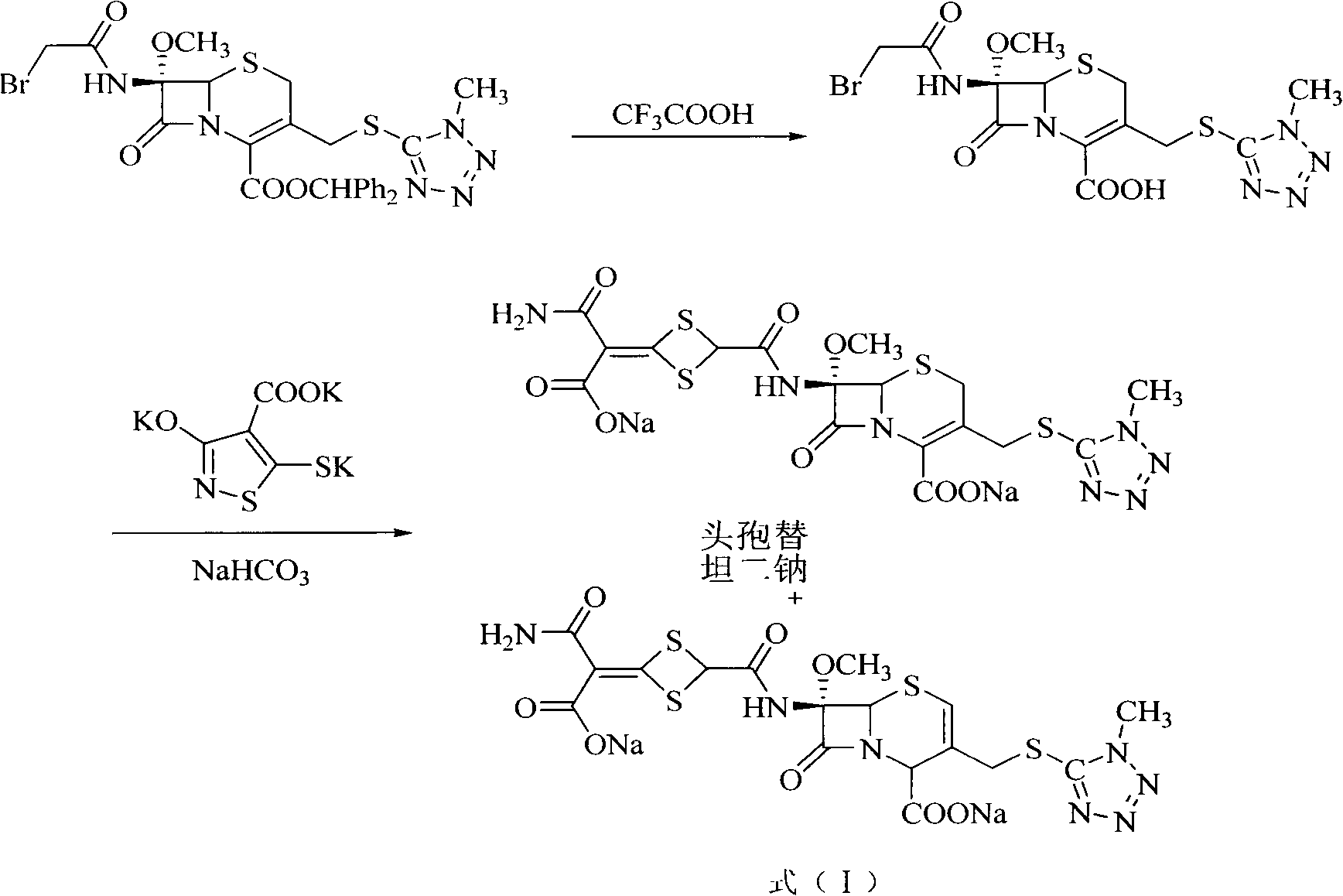
Method for preparing cefotetan bisodium
InactiveCN101050219ALow costSimple and fast operationOrganic chemistryCombinatorial chemistryCefotetan Disodium
This invention provides a method for preparing disodium cefotetan. The method has such advantages as abundant raw material (7-BAMCA), mild reaction conditions, no need for separation and purification of intermediate, and easy operation, thus is suitable for industrialization.
Owner:上海医药科技发展有限公司
Preparation method for cefotetan disodium
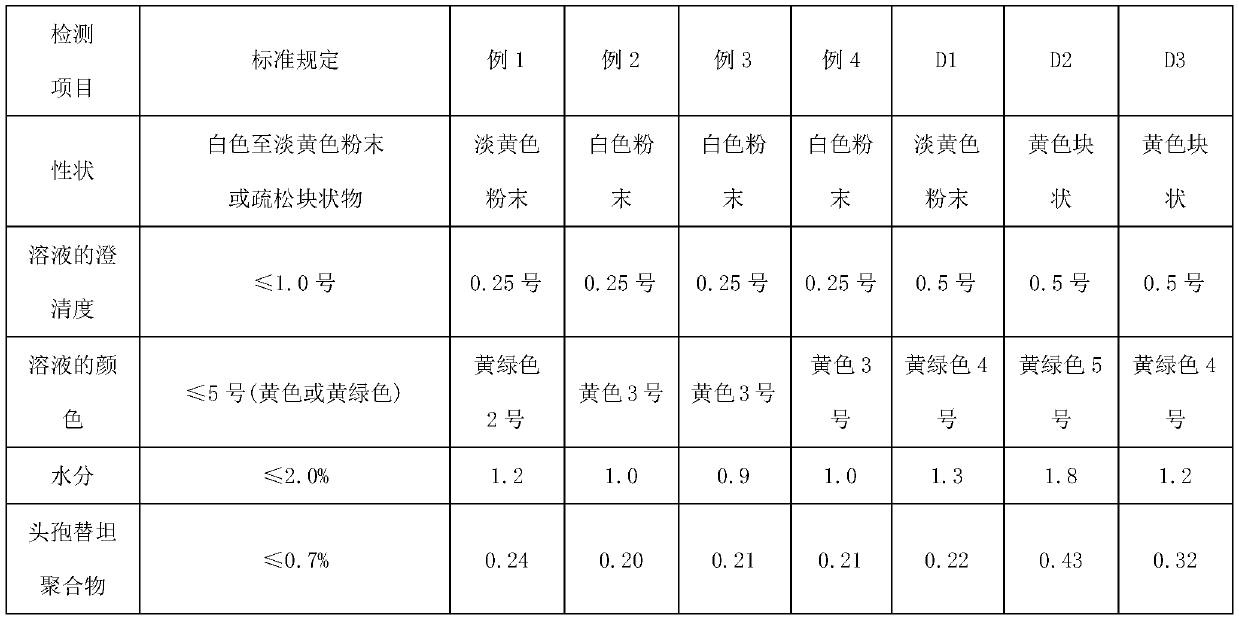
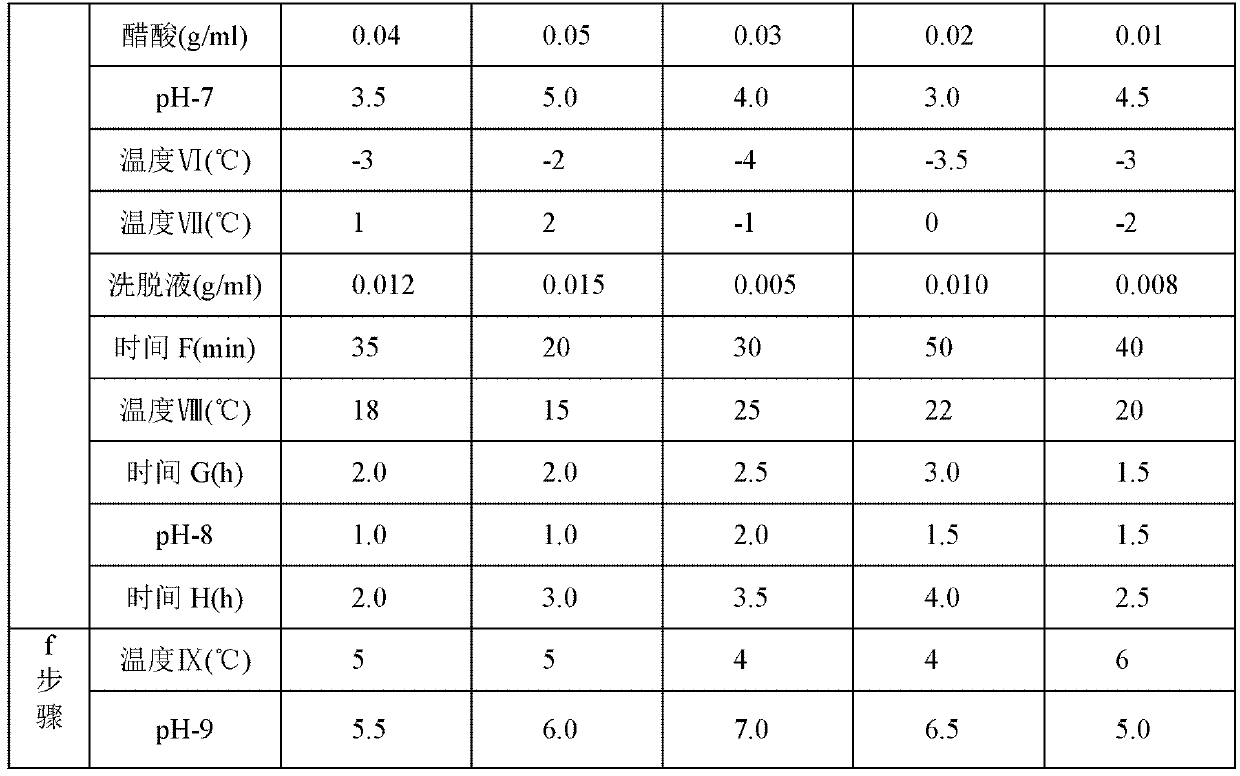
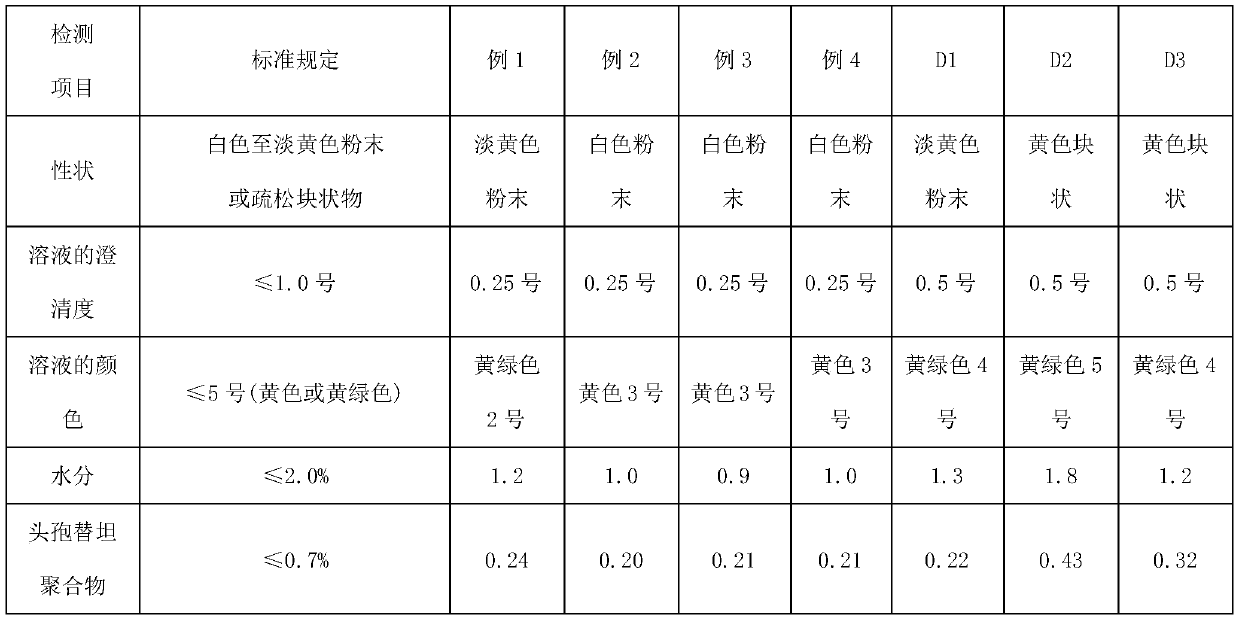
The invention relates to the technical field of medicine and particularly relates to a preparation method for cefotetan disodium. In the preparation method, cefotetan acid is mixed with silica gel through a first salifying, then a first filter liquor is collected through a first filtering, the first filter liquor is mixed with EDTA and active carbon, a second filter liquor is collected through filtering, then acid-forming and crystallization are performed, and then cefotetan acid product is obtained through third filtration, collection and filter cake, as well as washing; and the cefotetan acid product is taken to be mixed with active carbon after second salifying, fourth filter liquor is collected through the fourth filtering, and then degerming and freeze drying are performed, namely the cefotetan disodium is obtained. The product prepared by using the method provided by the invention is white, is excellent in solution clarity after solution, is less in the variety of residual solvent, is low in content of relevant matters such as polymer and the like, the adopted process is simple, the raw materials are easy to access, and the method is suitable for large-scale industrial production.
Owner:CHINA MEHECO SANYANG PHARMA CO LTD
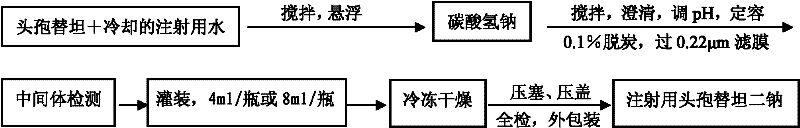
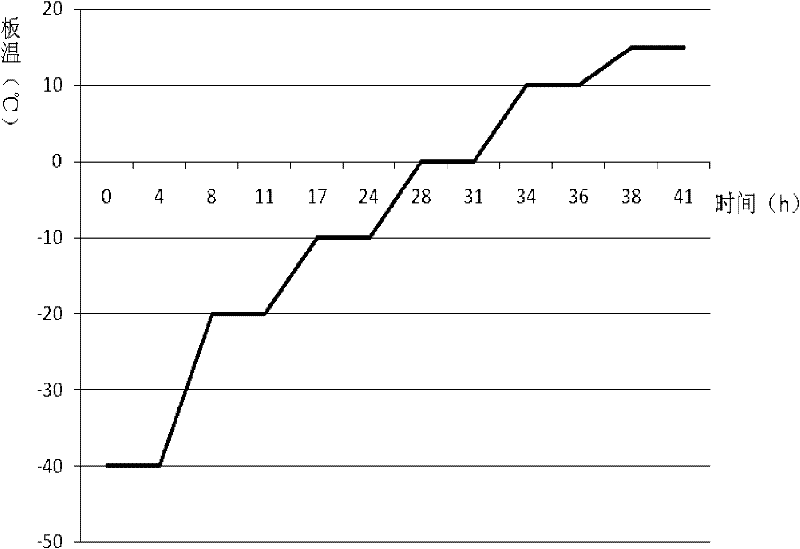
Cefotetan disodium for injection, and preparation method thereof
InactiveCN102247375AImprove stabilityReduce contentAntibacterial agentsPowder deliverySodium bicarbonateCefotetan Disodium
The invention relates to cefotetan disodium for injection, and a preparation method thereof. The cefotetan disodium for the injection is prepared from cefotetan, sodium bicarbonate, dilute hydrochloric acid and water for the injection, wherein the use amount of the sodium bicarbonate is 24.5-28.5% of the use amount of the cefotetan, preferably, 26.5%. The invention further provides the cefotetan disodium for the injection prepared from the cefotetan crystal, and the preparation method thereof. The cefotetan disodium for the injection provided by the present invention has good stability, small related substance content, such that the potential safety hazard problem due to large impurity content is avoided; the cefotetan disodium prepared from the cefotetan crystal provided by the present invention has good stability and small related substance content, meanwhile, because the prepared cefotetan disodium contains the cefotetan crystal, the prepared cefotetan disodium has strong antibacterial characterization.
Owner:从淑芳
Cefotetan disodium and preparation method of intermediate of cefotetan disodium
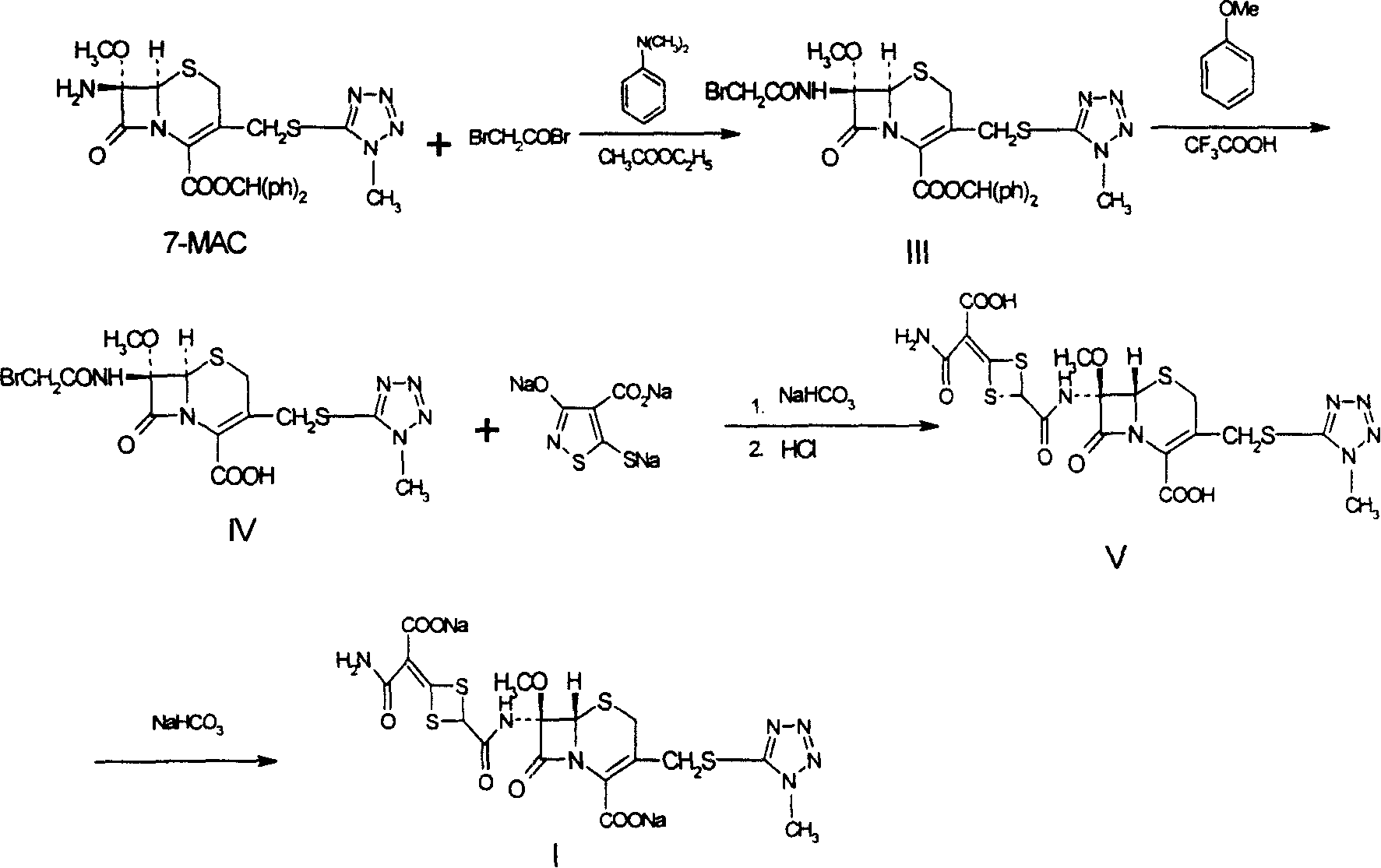
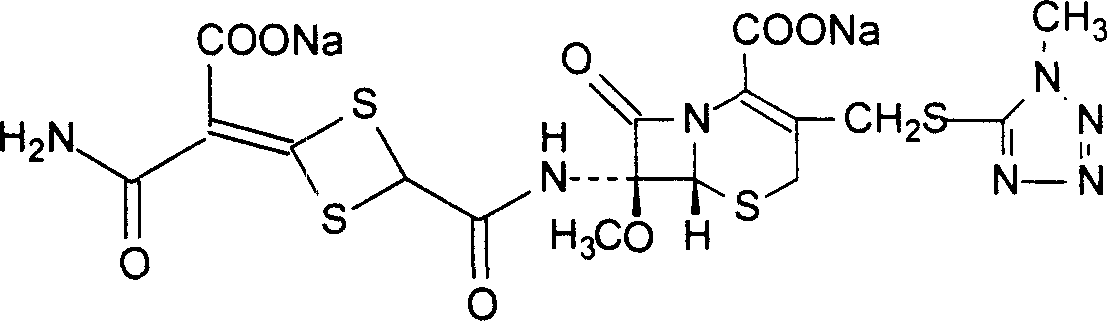
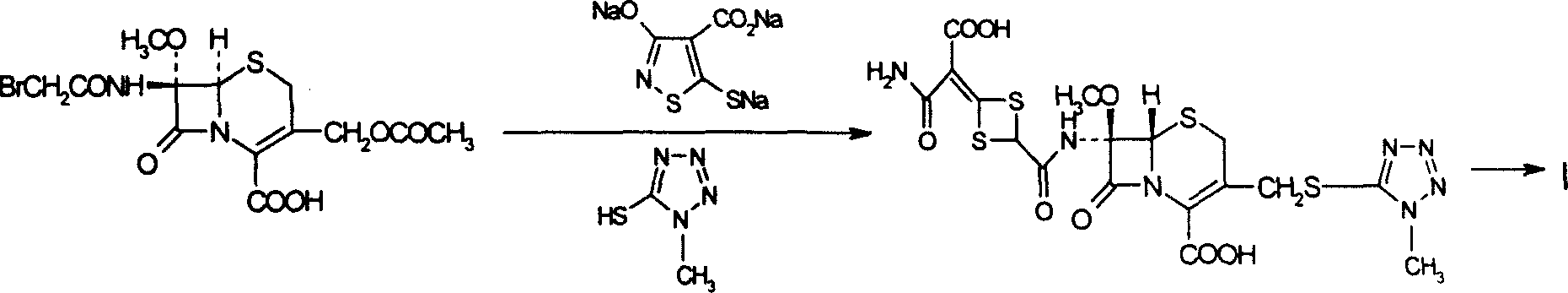
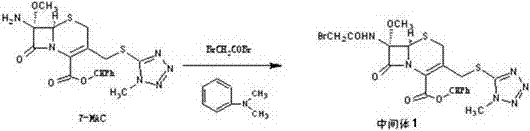
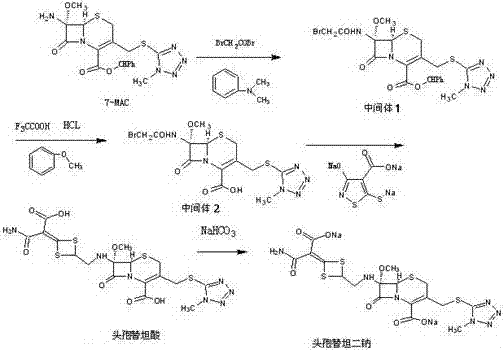
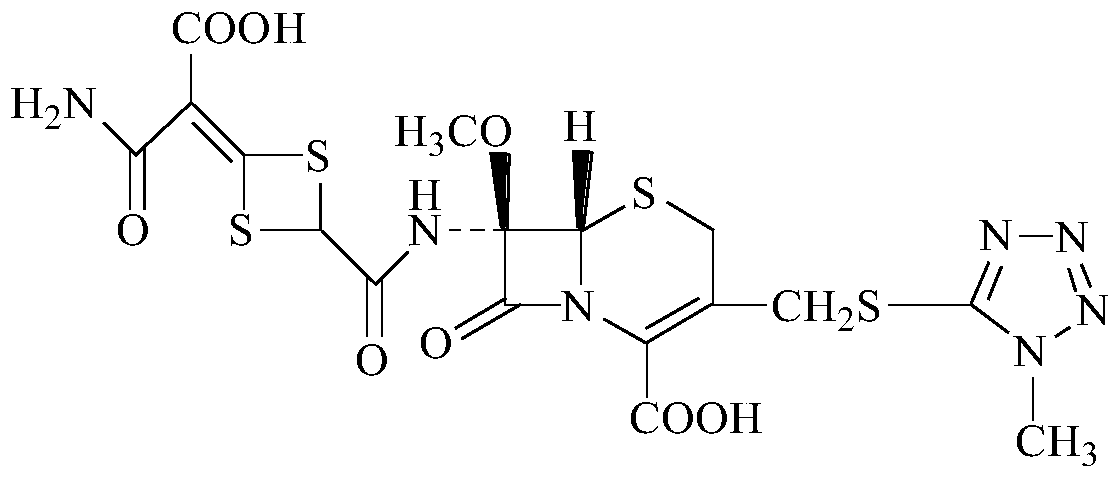
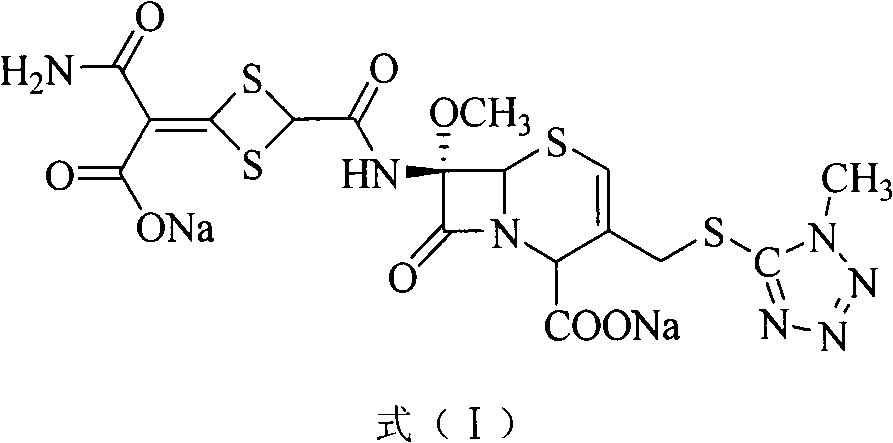
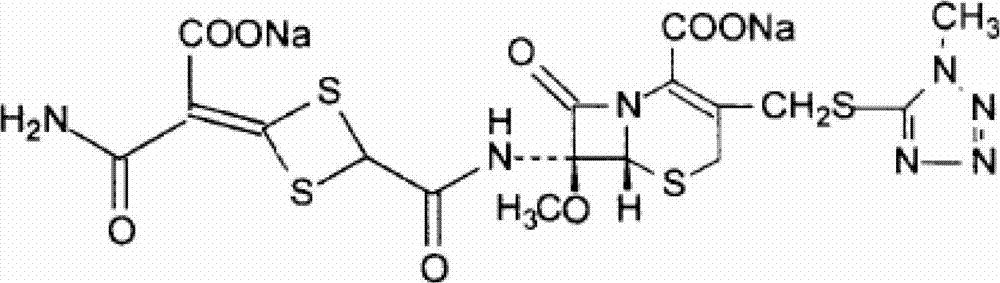
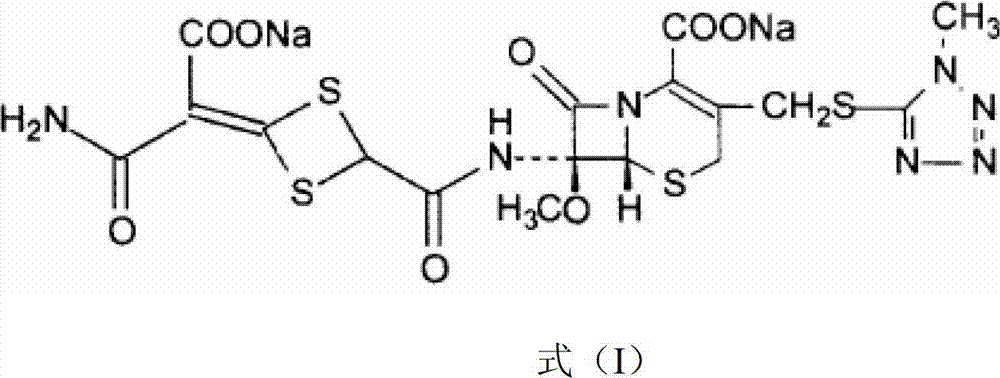
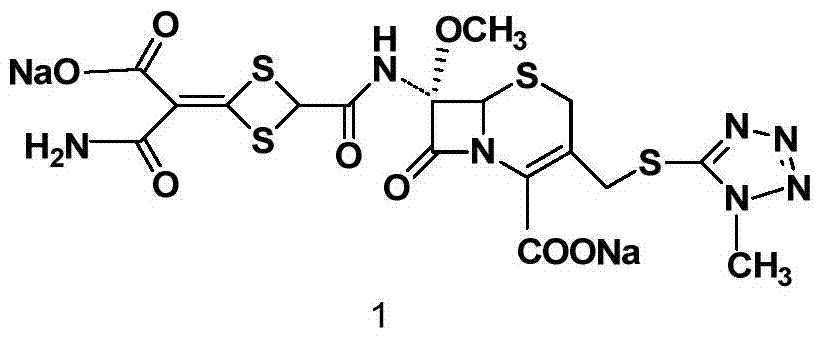
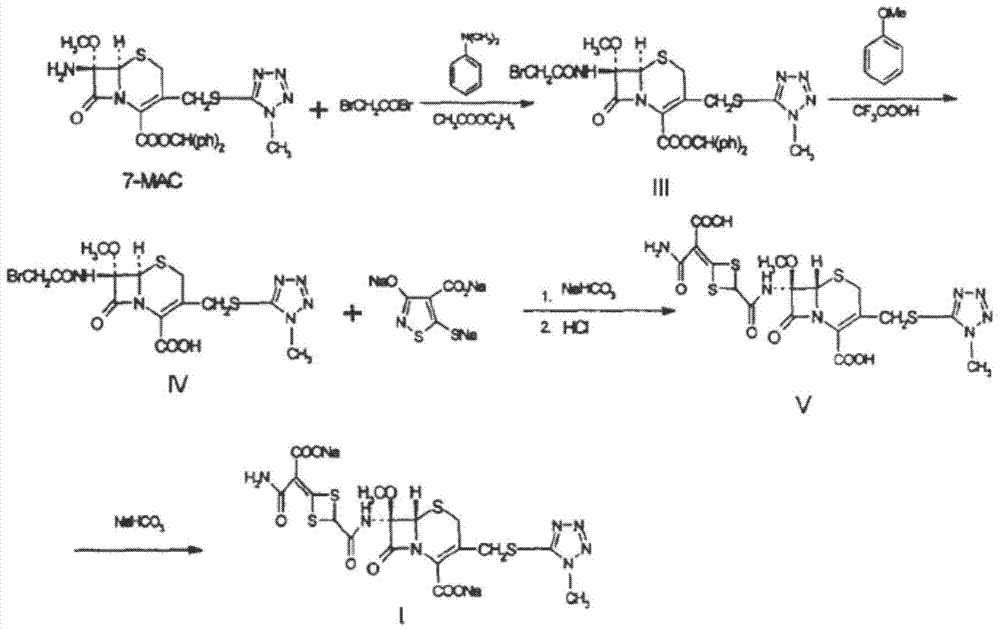
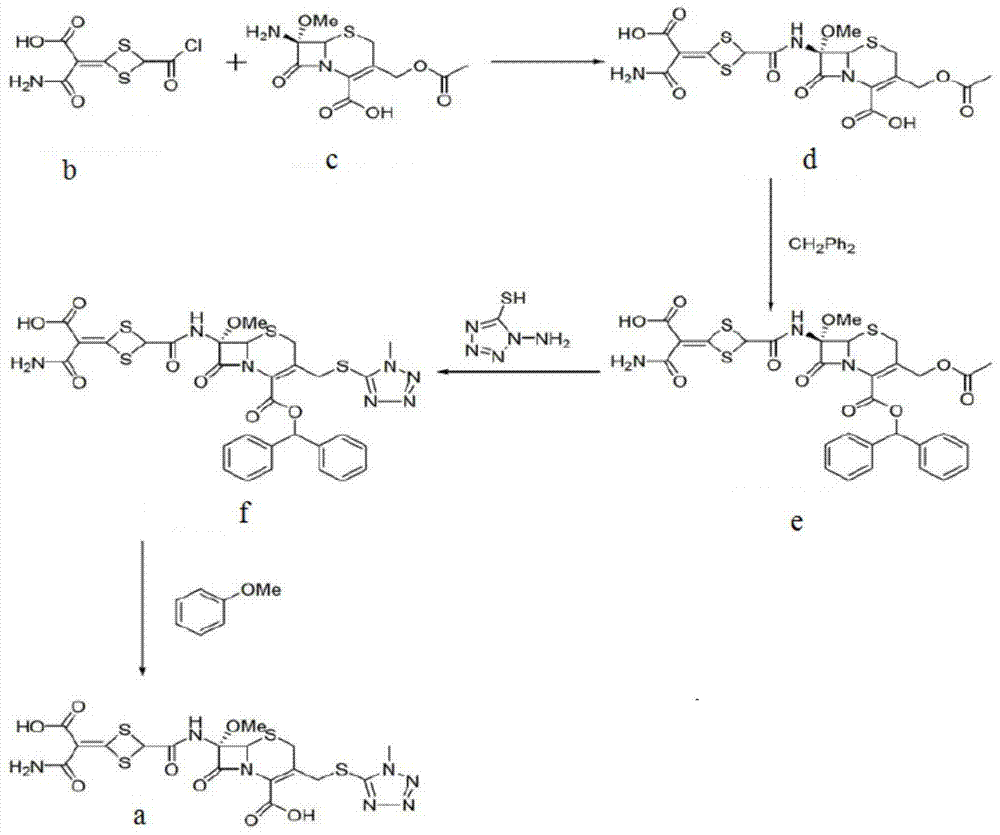
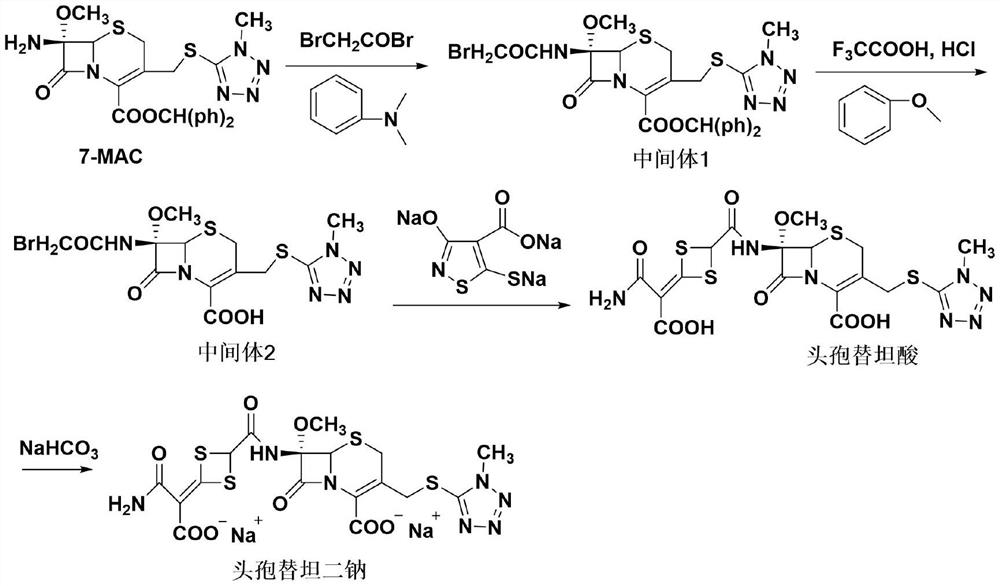
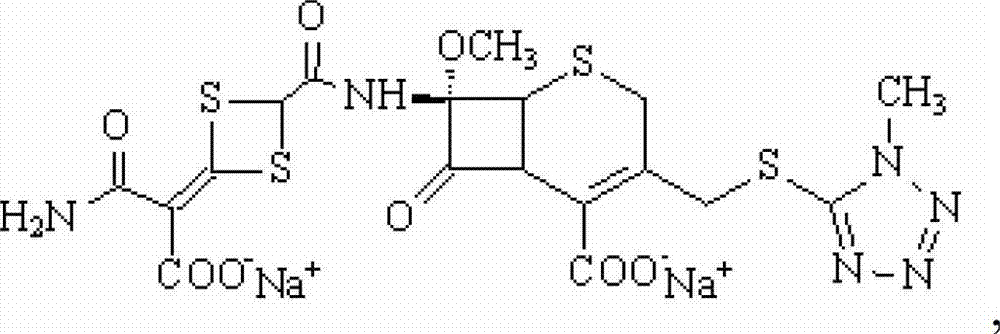
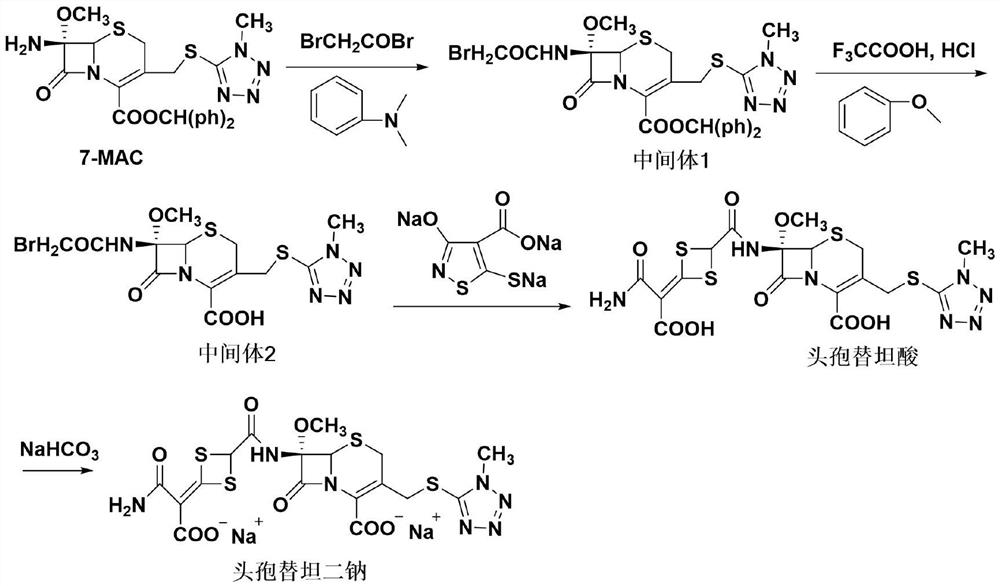
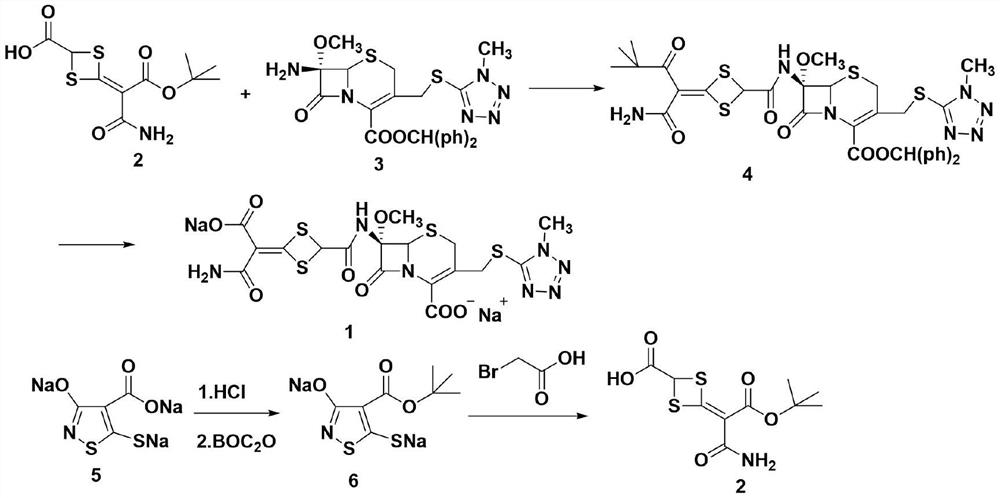
The invention discloses cefotetan disodium and a preparation method of an intermediate of cefotetan disodium. Commercially-available raw materials, namely, 4-(1-amino-3-tert-butoxy-1,3-dioxo-2-alkenyl)-1,3-dithietane-2-carboxylic acid (2) and 7-MAC (3) are subjected to a condensation reaction to obtain a compound (4), and then the compound (4) is subjected to deprotection and salification to obtain cefotetan disodium (1). The invention further provides a preparation method of the compound (2). In the route, no chloride agent contaminating the environment in a conventional route is used, and the preparation method has the advantages of being simple in process route and environmentally friendly, lowering the process cost and increasing the total product yield and is suitable for industrial production.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Cefotetan disodium hydrate and preparation method and application thereof
InactiveCN101838277AReduce humidityProcess stabilityAntibacterial agentsPowder deliveryDiseaseUpper urinary tract infection
The invention relates to a cefotetan disodium hydrate and a preparation method and application thereof. The cefotetan disodium hydrate has high storage stability, and is applicable to the preparation of medicines for treating or preventing diseases of the human being or animals caused by gram-positive or gram-negative sensitive bacteria, such as respiratory system diseases, hepatobiliary system diseases, five-sense organ diseases, urinary tract infection, celiac infection, pelvic infection, ichorrhemia, skin tissue infection, bone and joint infections, cephalomeningitis and endocarditis.
Owner:胡梨芳
Cephamycin intermediate compound and preparation method thereof
ActiveCN103193796ARaise the level of purityHigh purityOrganic compound preparationAmino compound preparationCEFMINOX SODIUMCarboxylic acid
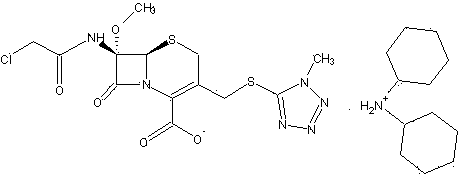
The invention relates to a cephamycin intermediate compound and a preparation method thereof. The cephamycin intermediate compound has a structure of formula (I), and is prepared by directly reacting 7beta-chloroacetamide-7alpha-methoxy-3-(1-methyl-1H-tetrazole-5-thiomethyl)-3-cephem-4-carboxylic acid and dicyclohexylamine in a specific solvent to form a salt and crystallizing. The cephamycin intermediate compound has the characteristics of high purity, high stability and the like, is conductive to improving the purity of cephamycin final products such as cefminox sodium, cefmetazole sodium and cefotetan disodium, and is beneficial to large-scale production and application.
Owner:山东安弘制药有限公司
Preparation method of cefotetan disodium
InactiveCN107540694AHigh purityLow impurity contentOrganic chemistryCefotetan DisodiumControllability
The invention discloses a preparation method of cefotetan disodium, comprising the following steps: (1) preparing a first intermediate; (2) separating by extraction; (3) preparing a second intermediate; (4) separating by crystallization; (5) preparing a cefotetan acid crude product; (6) purifying cefotetan acid; and (7) preparing cefotetan disodium. The preparation method of cefotetan disodium hasthe following advantages: the process is simple; reaction condition is mild; controllability of the reaction process is excellent; product yield is high; and the cefotetan disodium obtained has highpurity, low impurity content, good appearance quality and stable drug properties, and has a wide application prospect in clinic.
Owner:JIANGSU HI STONE PHARMA
Preparation method of cefotetan disodium
The invention discloses a preparation method of cefotetan disodium and belongs to the technical field of medicine. The preparation method includes the steps of A, adding methanol to a reactor by stirring and controlling the temperature, and then adding cefotetan disodium and salt-forming agent by stirring until dissolved clarification is achieved to obtain a mixed solution; B, controlling the temperature of the reactor and adding a small amount of organic solvent to the reactor till the system is a bit muddy for primary crystallization; C, after the completion of primary crystallization, adding an organic solvent for secondary crystallization, and performing filtration to obtain cefotetan disodium solid crystal; D, washing the cefotetan disodium solid crystal prior to atomizing drying andvacuum drying to obtain a cefotetan disodium product. The cefotetan disodium is low in the content of purities, good in fluidity, uniform in size distribution, stable in quality, convenient for subsequent sub-packaging of powder and needle preparation products and high in safety of drug use. The preparation method has the advantages of simpleness in operation and energy conservation and environment protection.
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA
Medicinal composition of cefotetan disodium
ActiveCN101590061AImprove stabilityPromote degradationAntibacterial agentsOrganic active ingredientsAdditive ingredientCefotetan Disodium
The invention discloses a medicinal composition of cefotetan disodium, which comprises the cefotetan disodium, and also comprises minor ingredients; the percentage by mass of the cefotetan disodium is 50 to 99 percent; and the percentage by mass of the minor ingredients is 1 to 50 percent. The medicinal composition of the cefotetan disodium is added with the minor ingredients capable of strengthening the stability of the cefotetan disodium to inhibit the quick degradation of the cefotetan disodium when the cefotetan disodium meets water in the processes of production, storage and use and improve the stability of the cefotetan disodium, so that a high-efficiency and stable medicinal composition product of the cefotetan disodium is obtained.
Owner:SHENZHEN LIJIAN PHARM CO LTD
Amorphous cefotetan acid, method for preparing cefotetan disodium by amorphous cefotetan acid and pharmaceutical composition containing cefotetan disodium
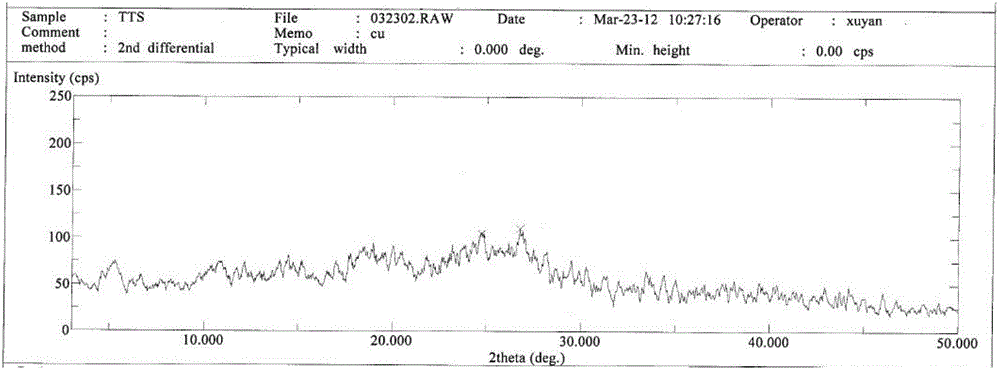
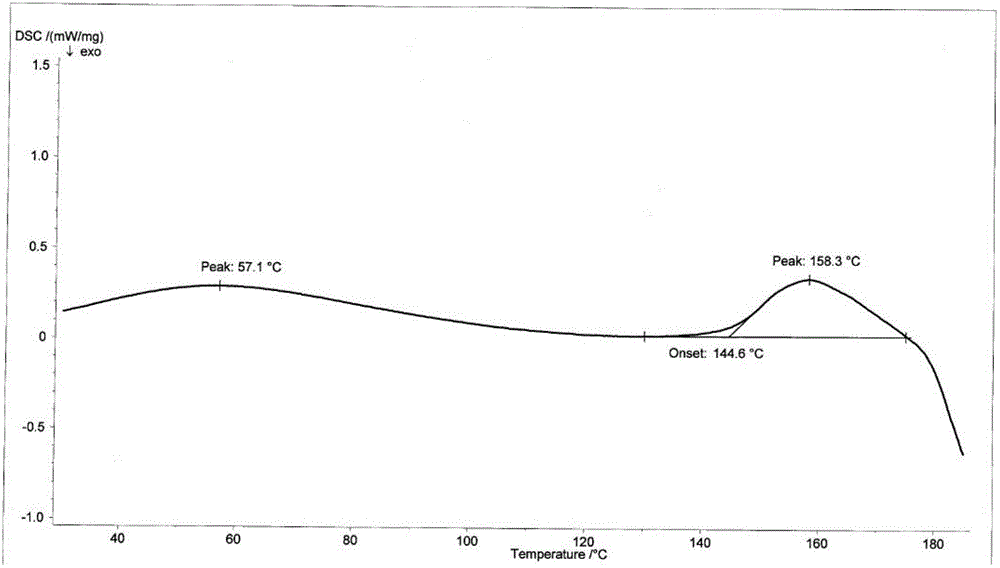
InactiveCN103724359AImprove solubilityTroubleshoot unstable technical issuesAntibacterial agentsOrganic active ingredientsOrganic solventX-ray
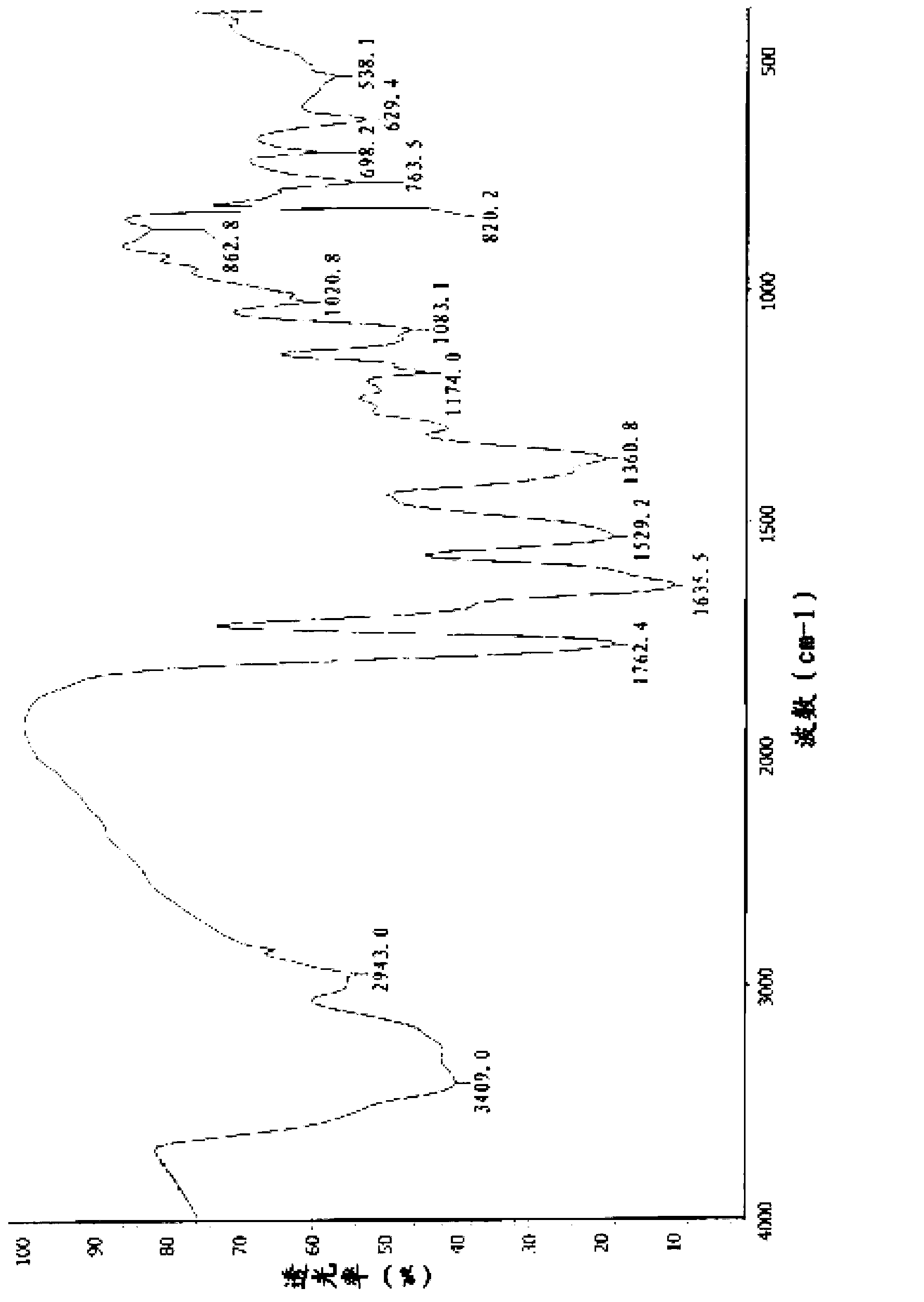
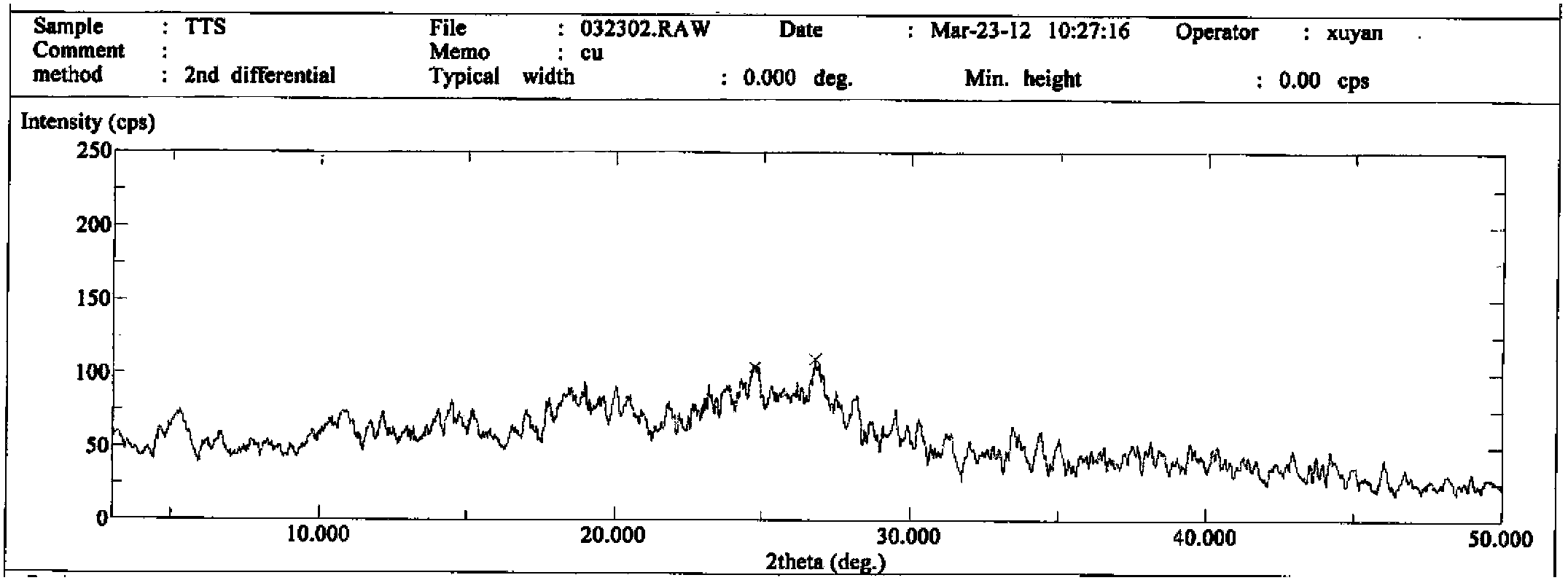
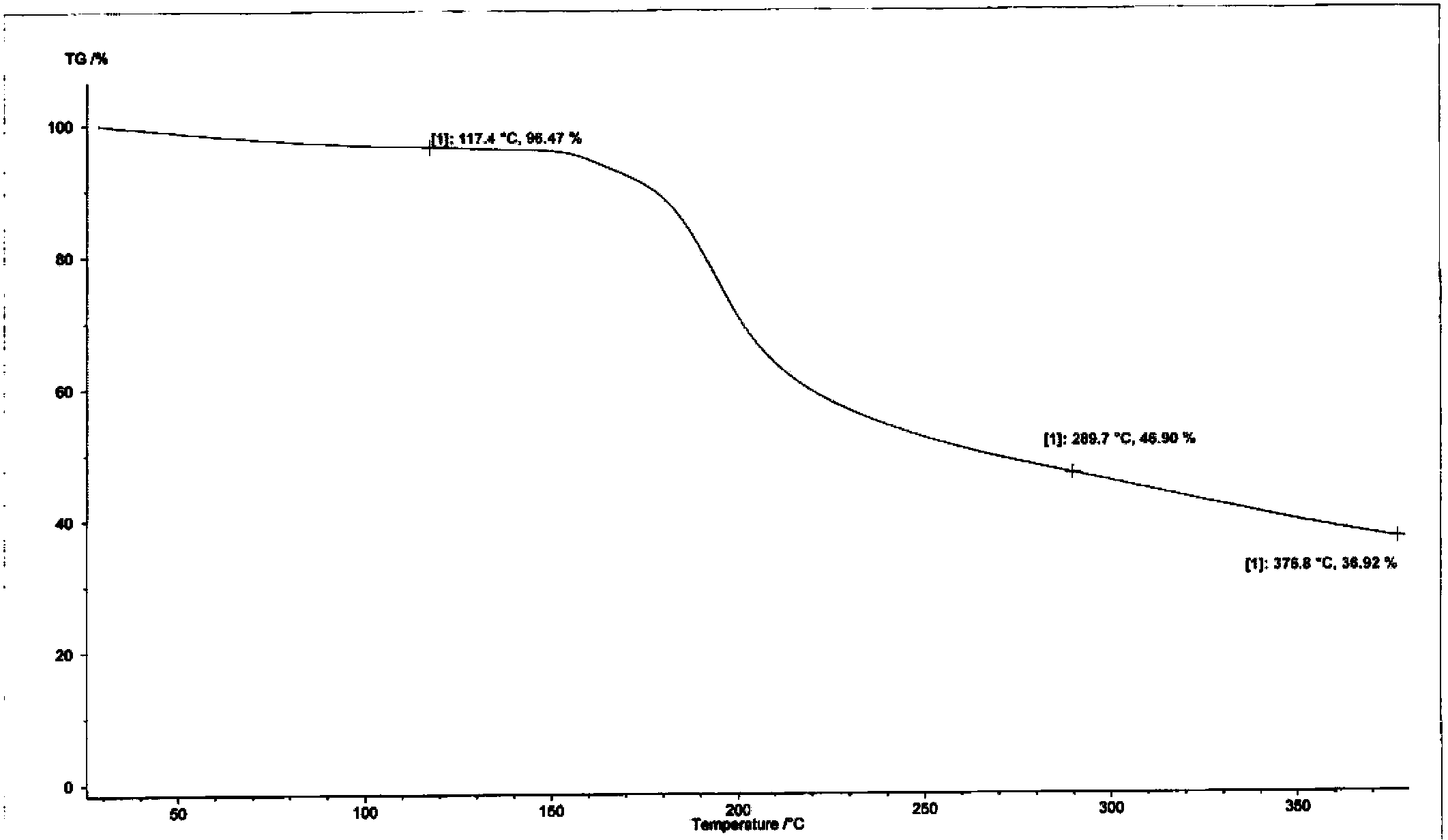
The invention discloses a novel crystal form of cefotetan acid, a method for preparing cefotetan disodium by the novel crystal form of the cefotetan acid and a pharmaceutical composition containing the cefotetan disodium. The X-ray diffraction pattern of the novel crystal form appears no X-ray diffraction peak; the endothermic peak in the differential thermal analysis pattern is positioned at 158.3 DEG C; the exothermic peak is positioned at 144.6 DEG C. The novel crystal form solves the technical problem of instability of the cefotetan disodium prepared by using the cefotetan acid in the prior art. The used preparation method has good operability and few residues of organic solvent, and is specifically suitable for large-scale industrial production.
Owner:SHENZHEN SALUBRIS PHARMA CO LTD
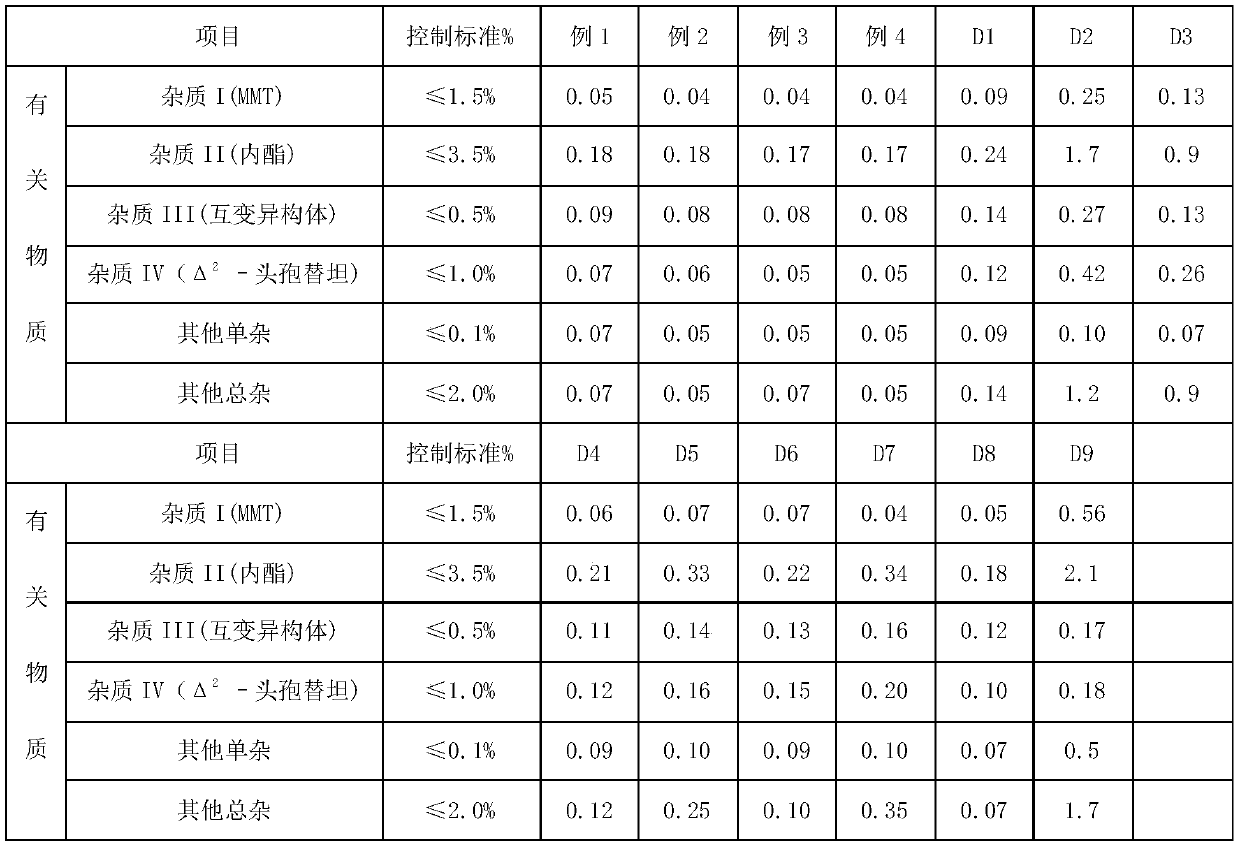
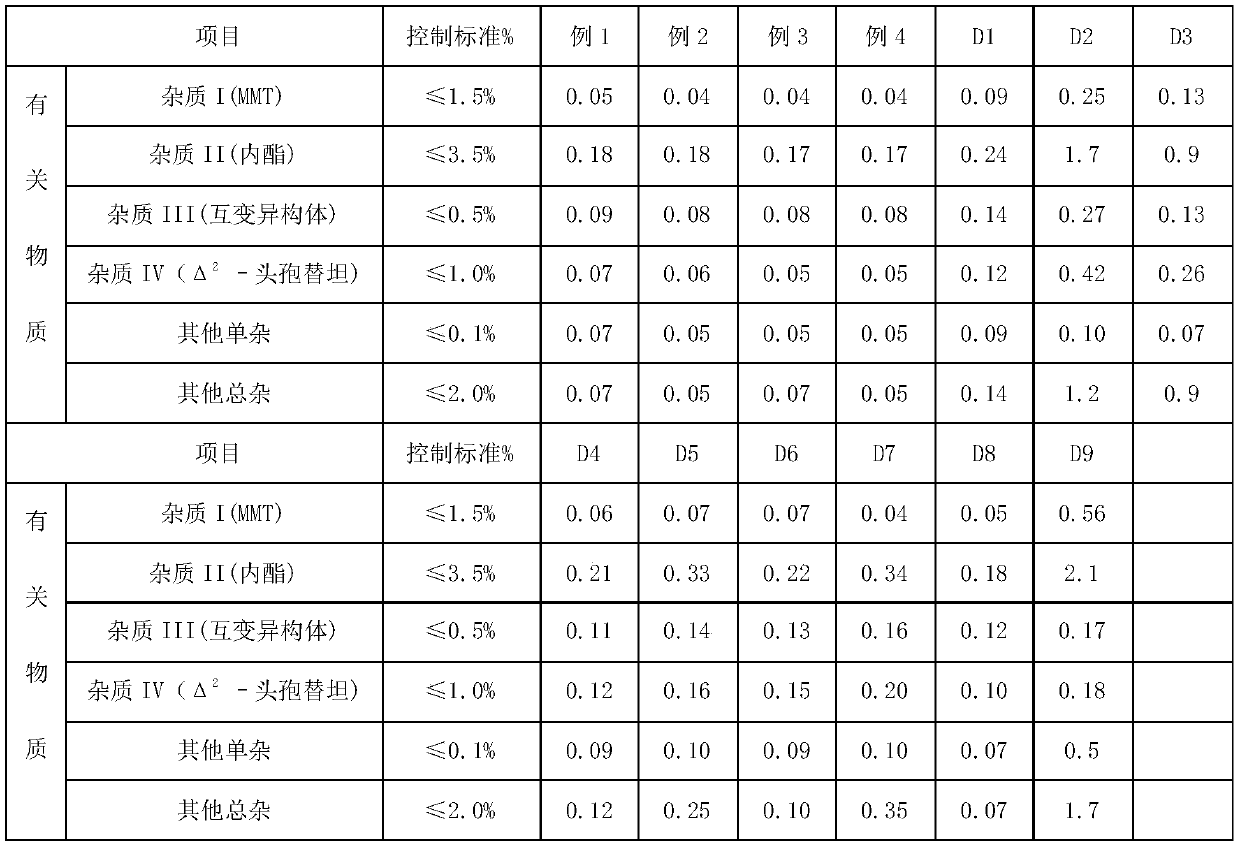
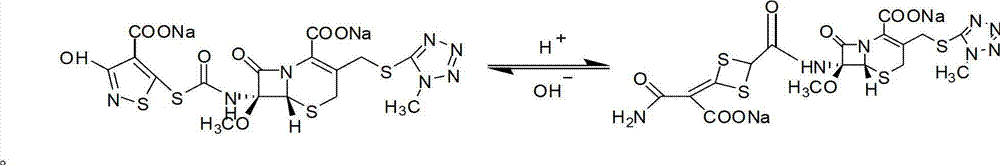
Method for removing impurity delta<2>-isomer from cefotetan disodium
InactiveCN102161669AShort reaction timeHigh reaction yieldOrganic chemistrySodium bicarbonateThermal insulation
The invention relates to a method for removing an impurity, namely delta<2>-isomer from cefotetan disodium. Specifically, the method comprises the following steps of: adding crude cefotetan disodium, copper (I) hydride and tri-n-butyl tin hydride into acetonitrile, stirring uniformly under the protection of nitrogen, reacting under thermal insulation, pouring the obtained product into 5 percent diluted hydrochloric acid, extracting an aqueous phase by using ethyl acetate, mixing ethyl acetate phases, washing the merged ethyl acetate phase by using water, drying by using anhydrous sodium sulfate, filtering, concentrating ethyl acetate under reduced pressure, treating by using sodium bicarbonate, and filtering to obtain the cefotetan disodium. The yield is up to 94 percent.
Owner:JUMPCAN PHARMA GRP
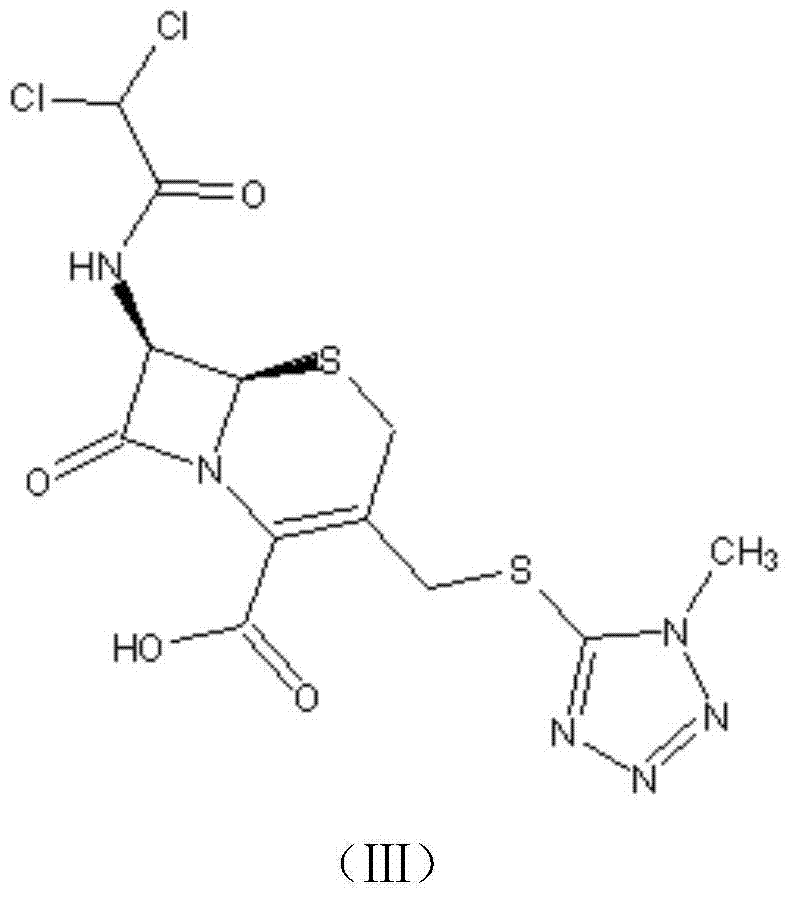
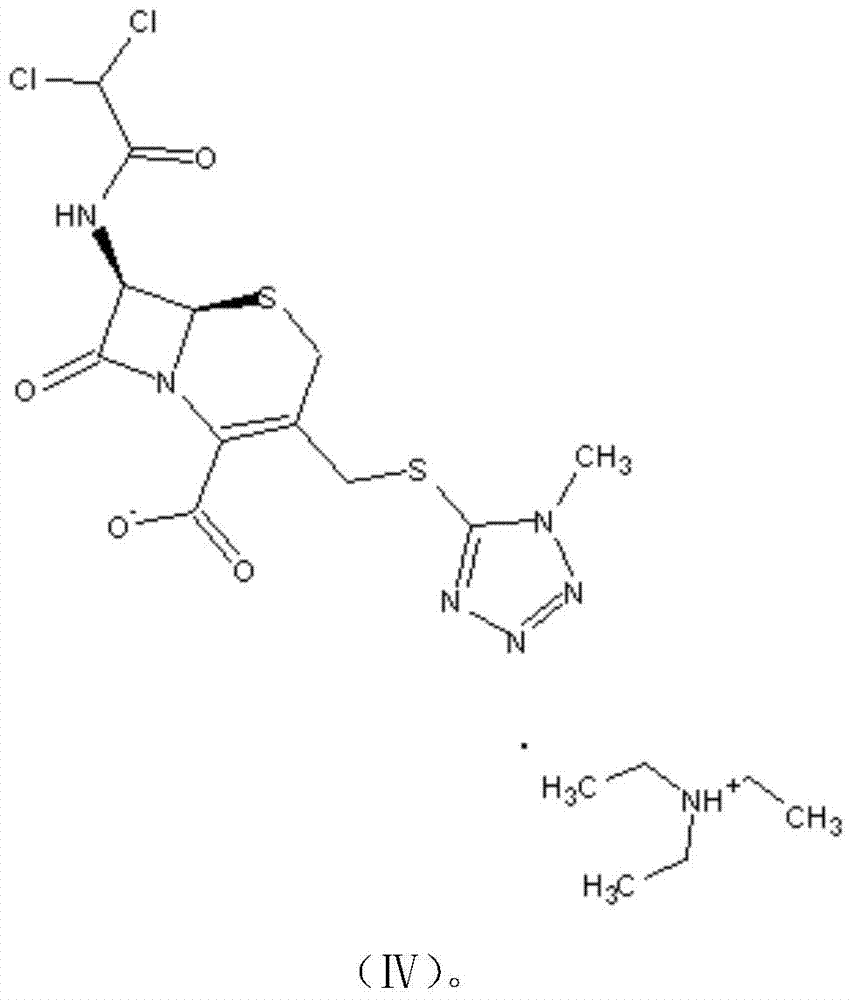
Preparation method for cephamycin intermediate
The invention relates to a preparation method for a cephamycin intermediate. A compound of the cephamycin intermediate can be seen in the formula (IV). The cephamycin intermediate is obtained by making 7-ACA react with dichloroacetyl chloride in specific solvent, making the obtained product react with MMT under catalysis of a sulfoacid catalyst and conducting crystallization. The cephamycin intermediate has the advantages of being high in purity and stability and the like, is beneficial for improving the purity of final cephamycin products such as cefminox sodium, cefmetazole and cefotetan disodium, and is beneficial to large-scale production and application.
Owner:山东安弘制药有限公司
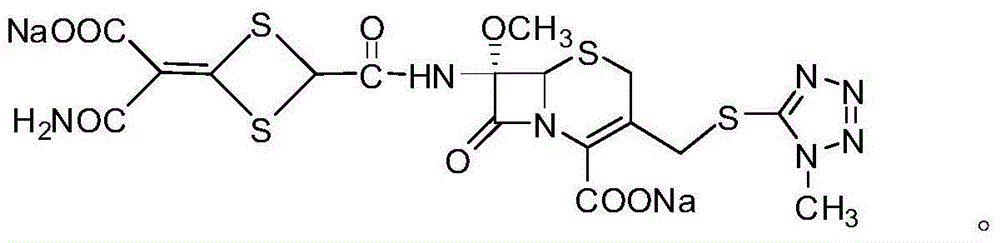
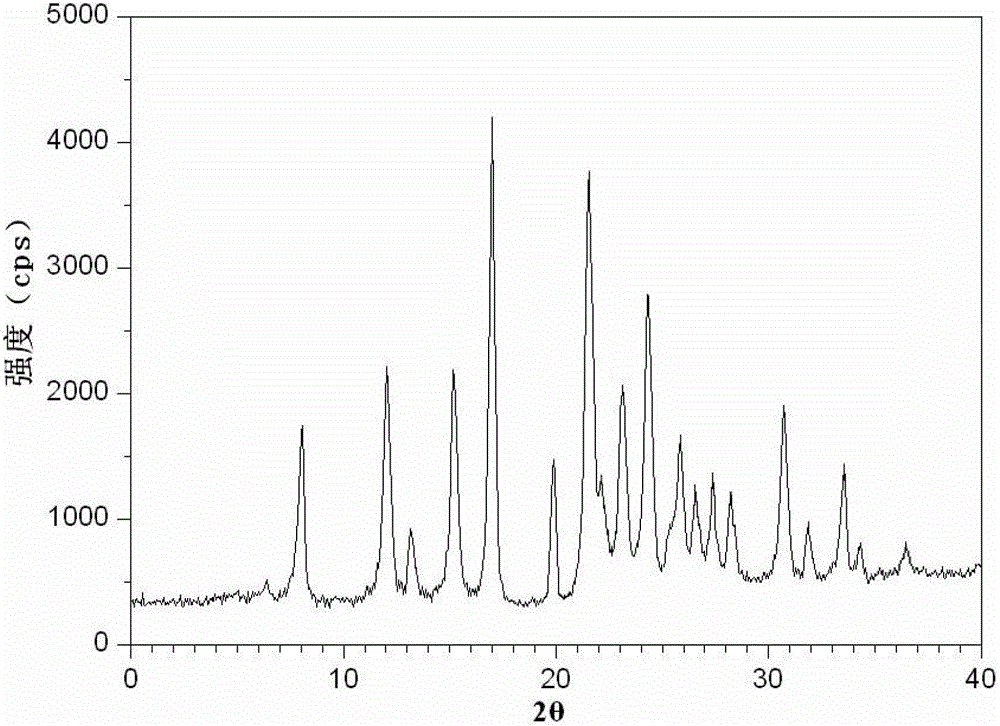
Cefotetan disodium compound as well as preparation method and medicinal composition thereof
ActiveCN102731532AExcellent acceleration stabilityProcess stabilityAntibacterial agentsOrganic active ingredientsChemistryCefotetan Disodium
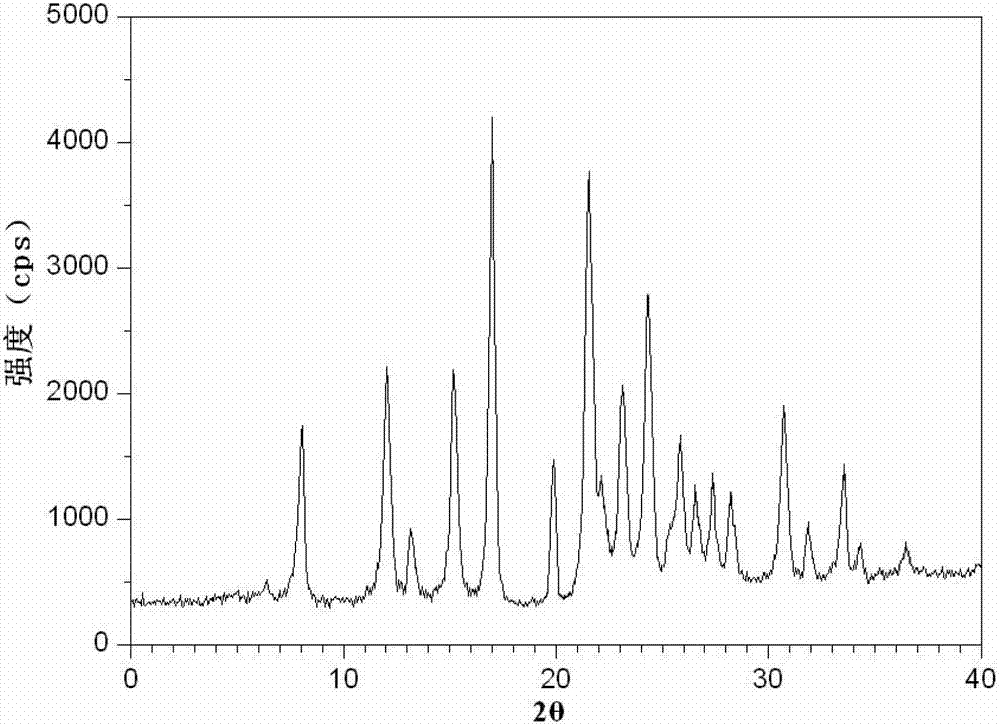
The invention relates to a preparation method and a medicinal composition of a cefotetan disodium compound. The cefotetan disodium compound is crystal, wherein characteristic peak of the crystal in an X-ray powder diffraction pattern measured by using Cu-Ka ray is indicated in 2 theta of 8.0 degrees, 12.1 degrees, 15.4 degrees, 17.0 degrees, 19.8 degrees, 21.6 degrees, 23.0 degrees, 24.3 degrees, 25.7 degrees, 27.4 degrees, 30.7 degrees and 33.5 degrees. The compound crystal has good stability and hygroscopicity, and can be well stored and saved, so that the stability of cefotetan disodium is improved to a certain degree.
Owner:永春县产品质量检验所福建省香产品质量检验中心国家燃香类产品质量监督检验中心福建
A kind of preparation method of cefotetan disodium and intermediate thereof
Owner:SHANDONG LUOXIN PHARMA GRP CO LTD
Preparation method of cefmetazole
The invention relates to a preparation method of cefmetazole. The preparation method comprises the steps of adopting a one-pot method containing 7-ACA, 1-methyl-5-tetrazole-thione and chloroacetyl chloride, obtaining 7-DCT, then using seven-position methoxy groups of sodium methylate and tert-butyl hypochlorite, and obtaining methoxy cephalosporin mother nuclei which are shared mother nuclei. The cefmetazole can serve as four methoxy cephalosporin products, namely cefminox, cefmetazole, cephalosporin and cefotetan disodium, three steps are omitted, the processing steps are greatly shortened, cost is lowered, and the overall yield is raised to 70%.
Owner:NANJING JINHAO MEDICAL TECH CO LTD
Preparation of impurity in cefetecol disodium and structure confirmation method
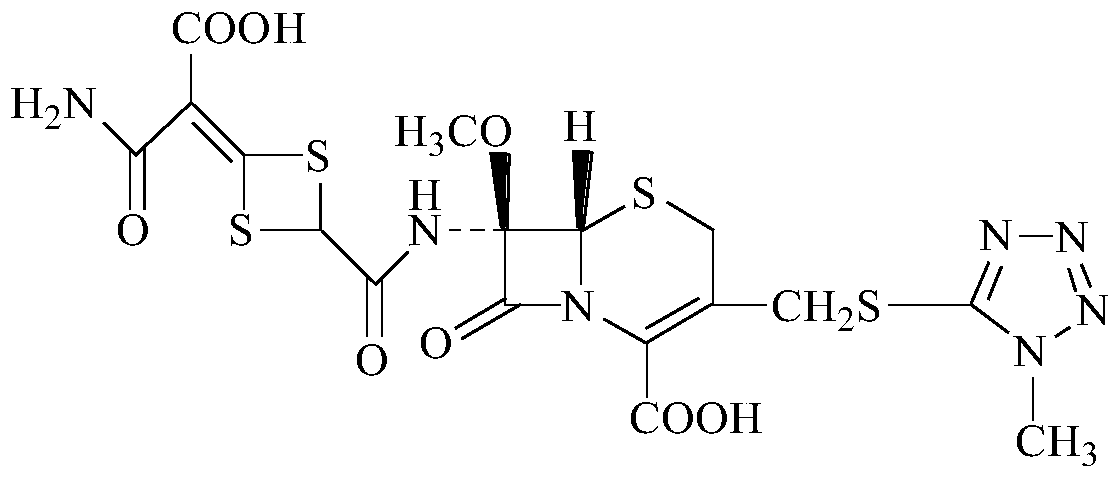
ActiveCN104910192AConfirmed chemical structureSimple methodOrganic chemistryChemical structureCefotetan Disodium
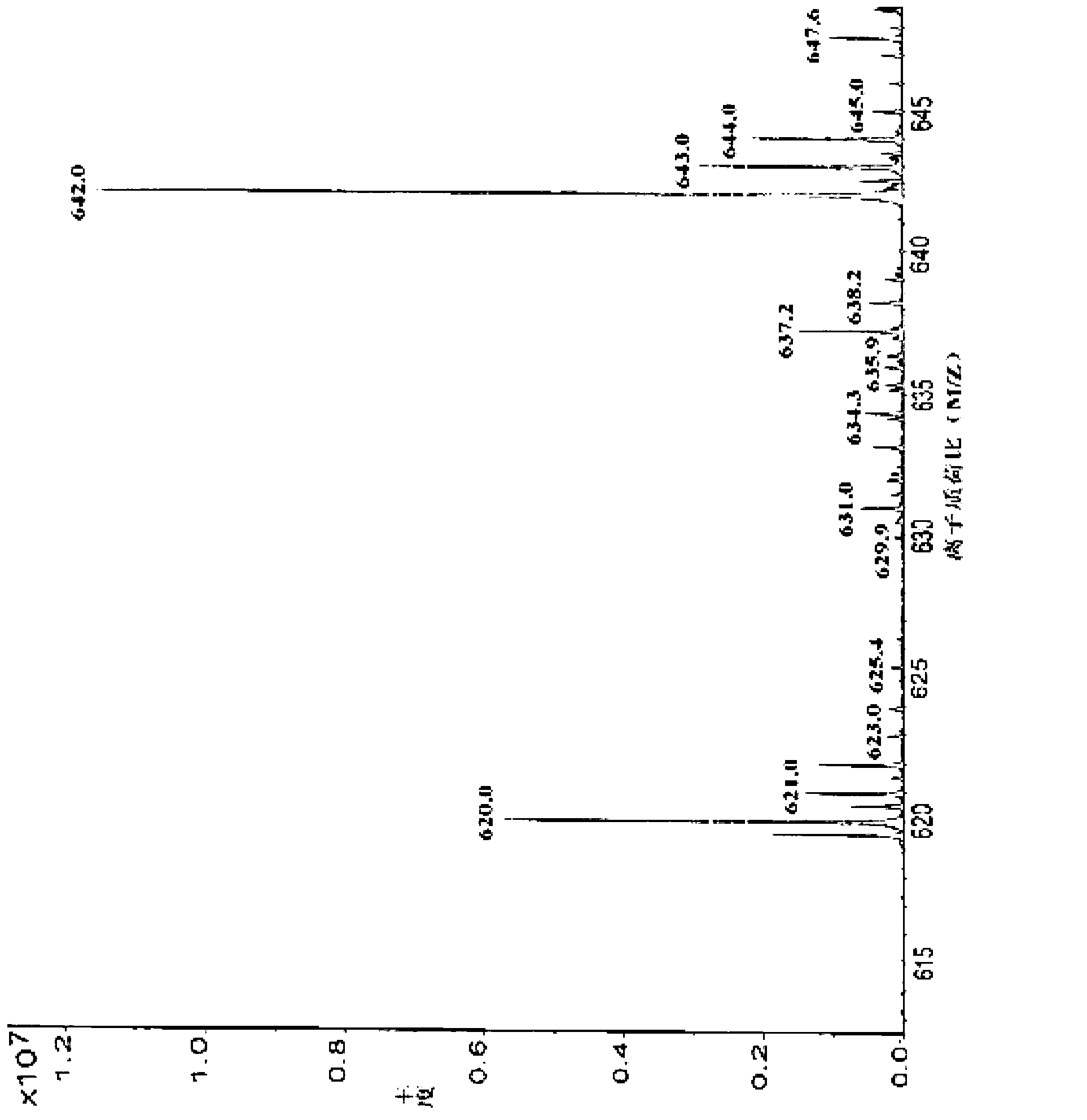
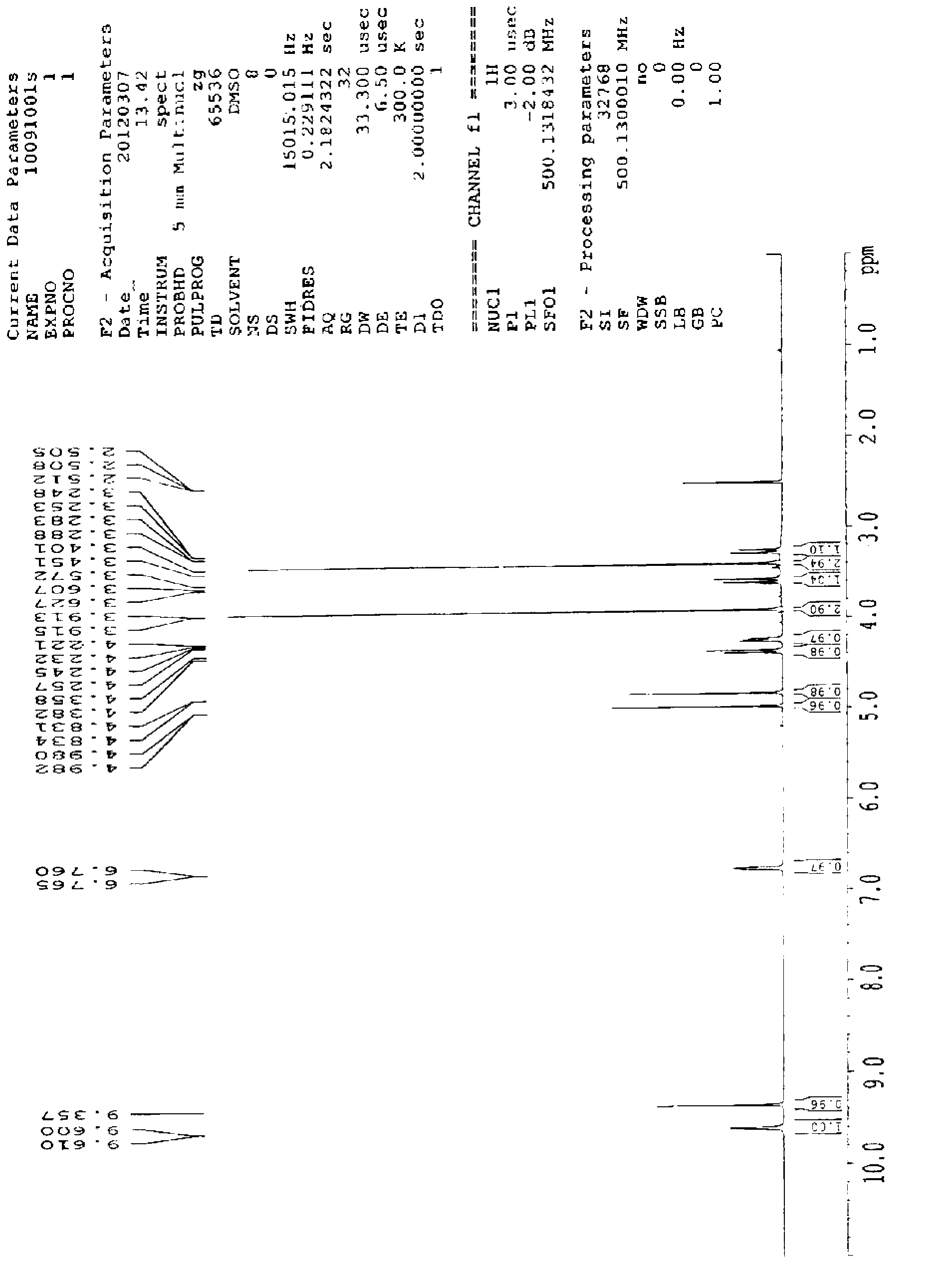
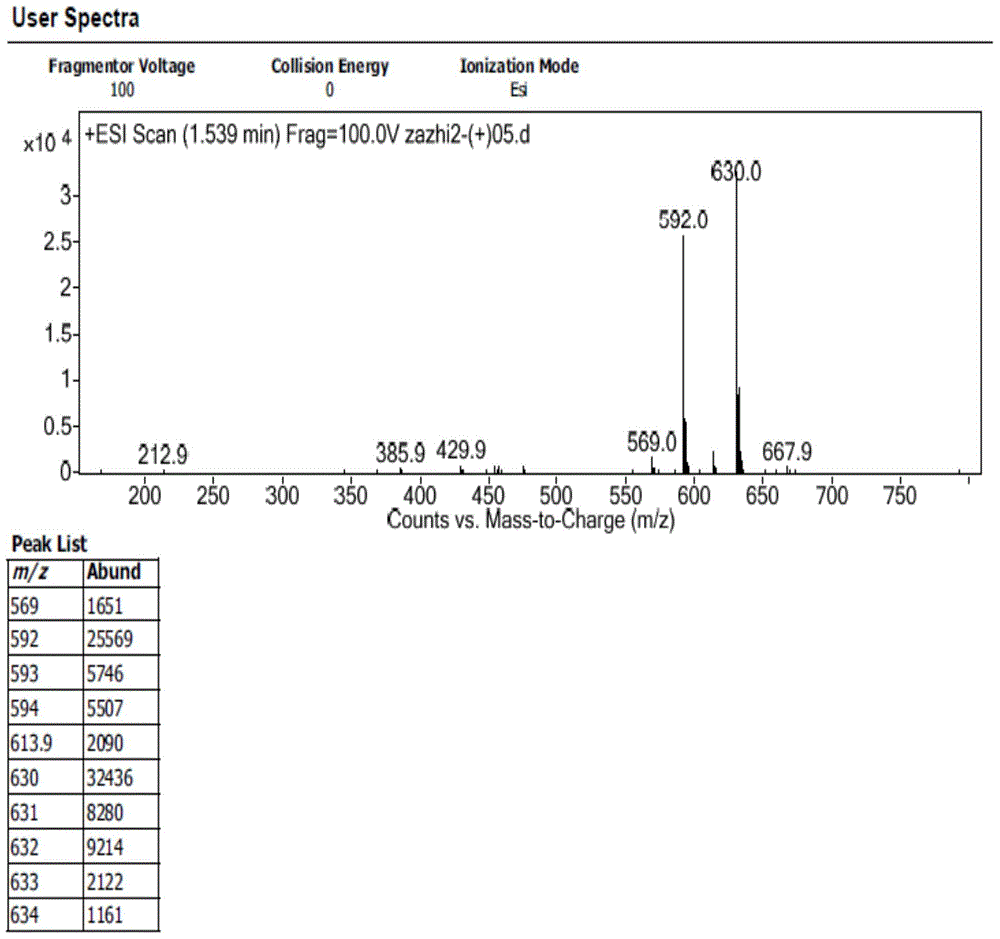
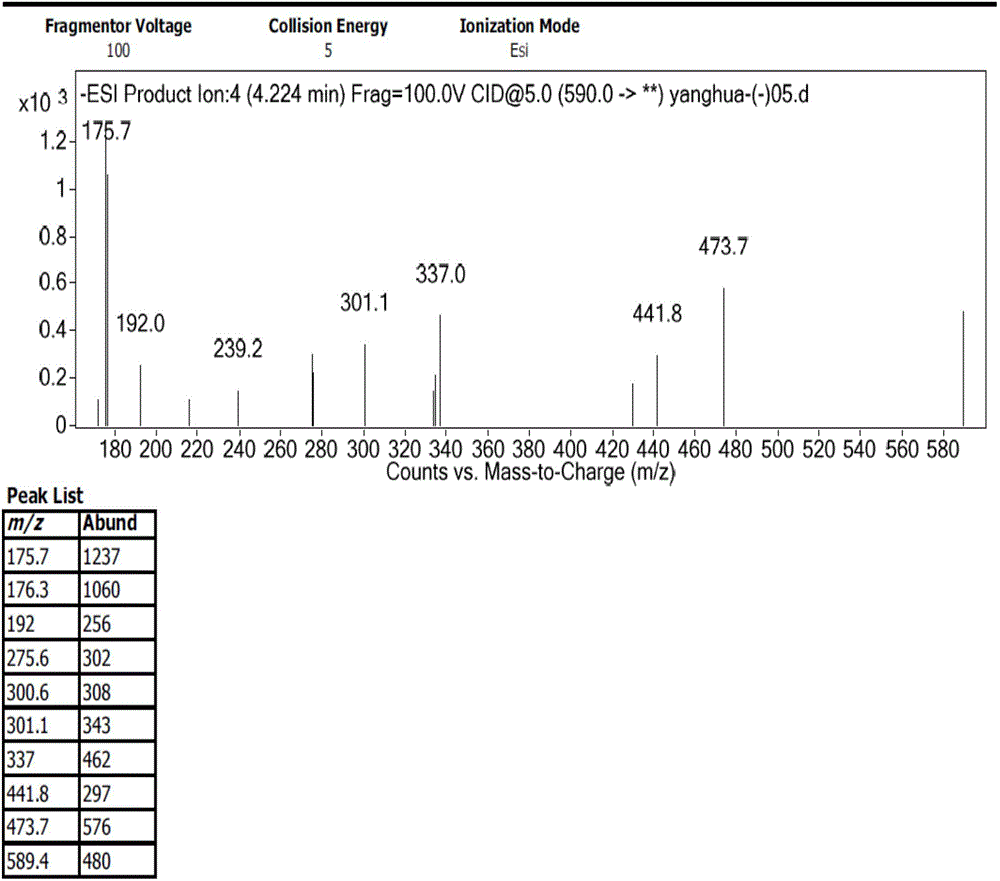
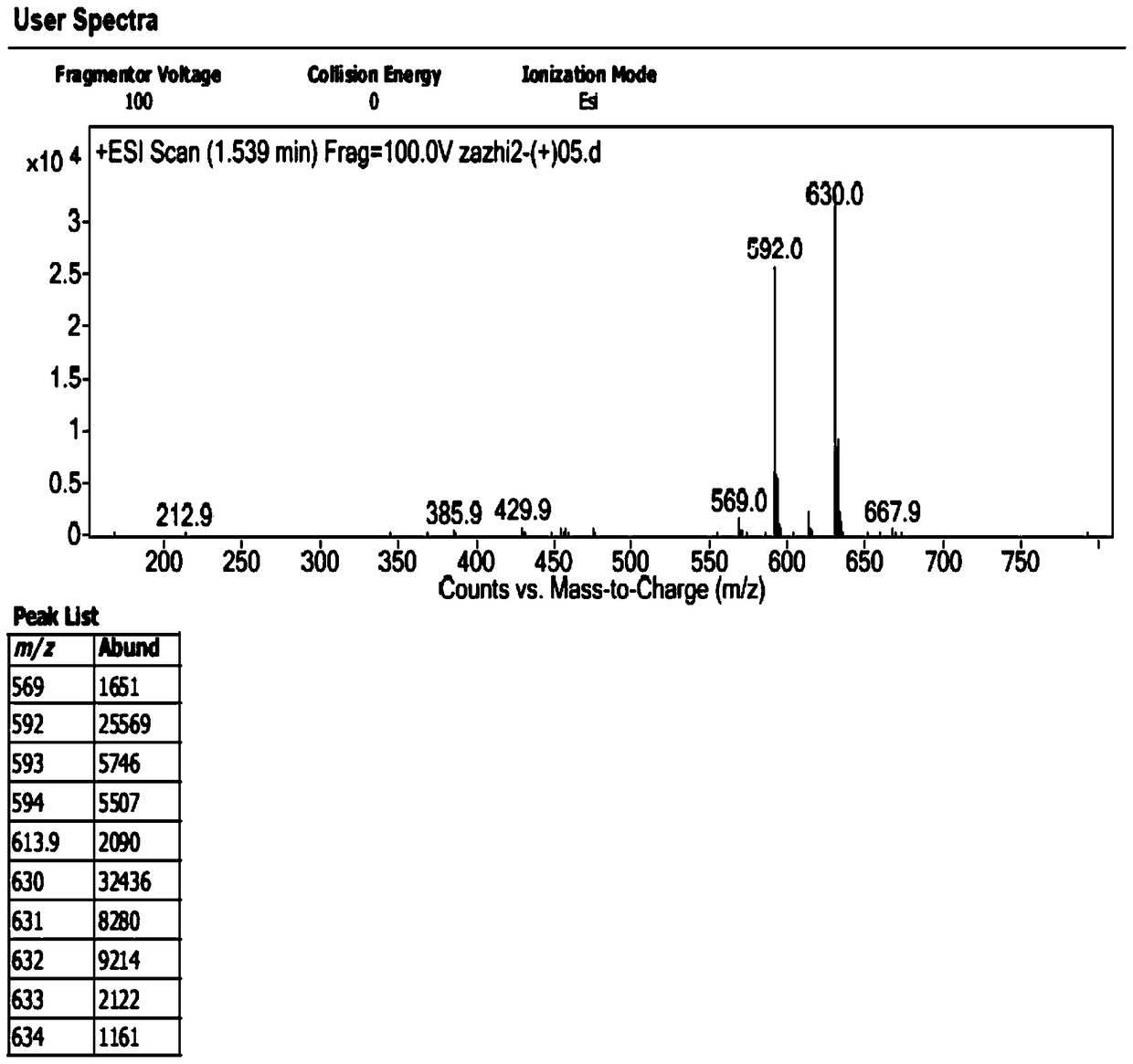
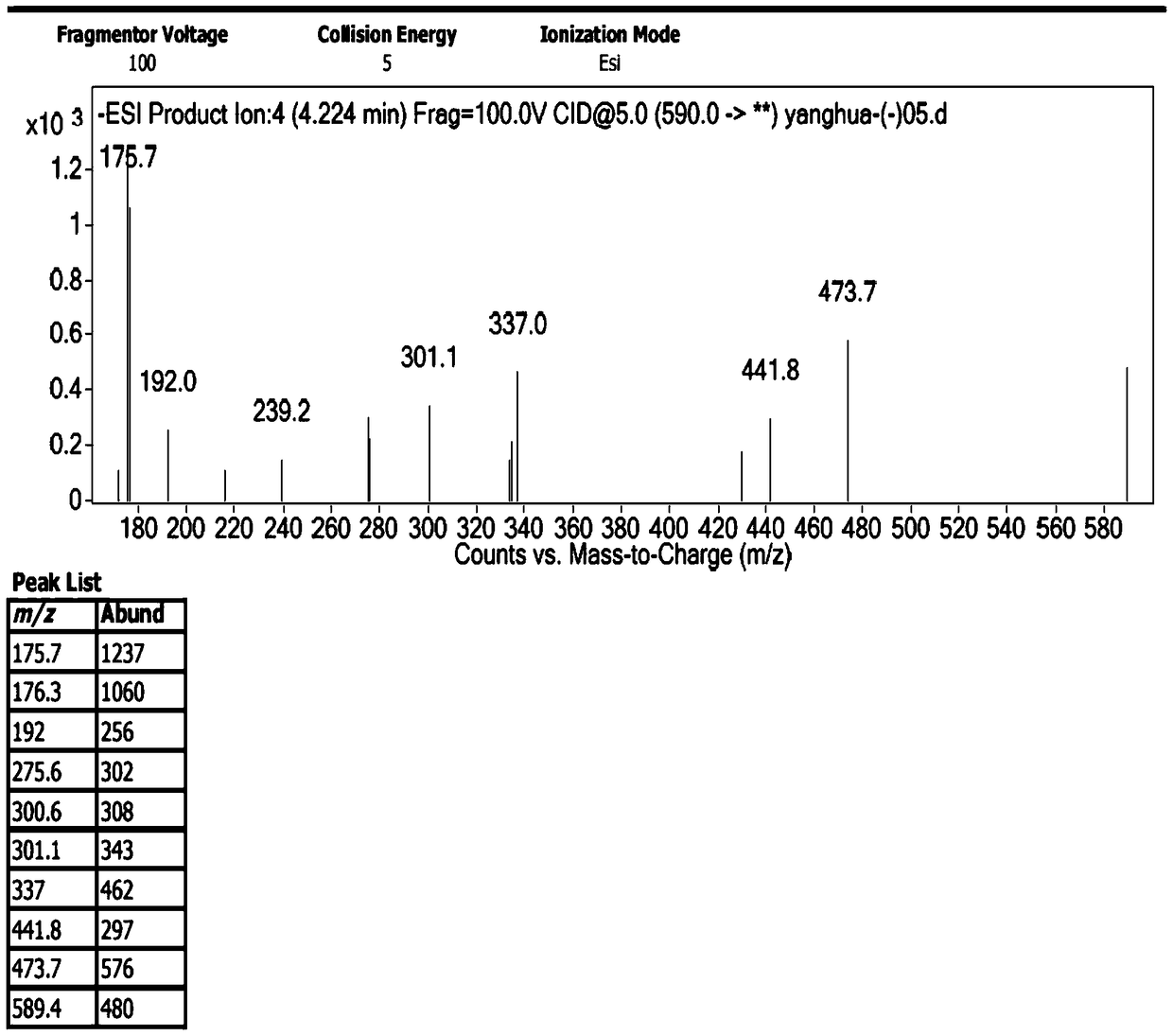
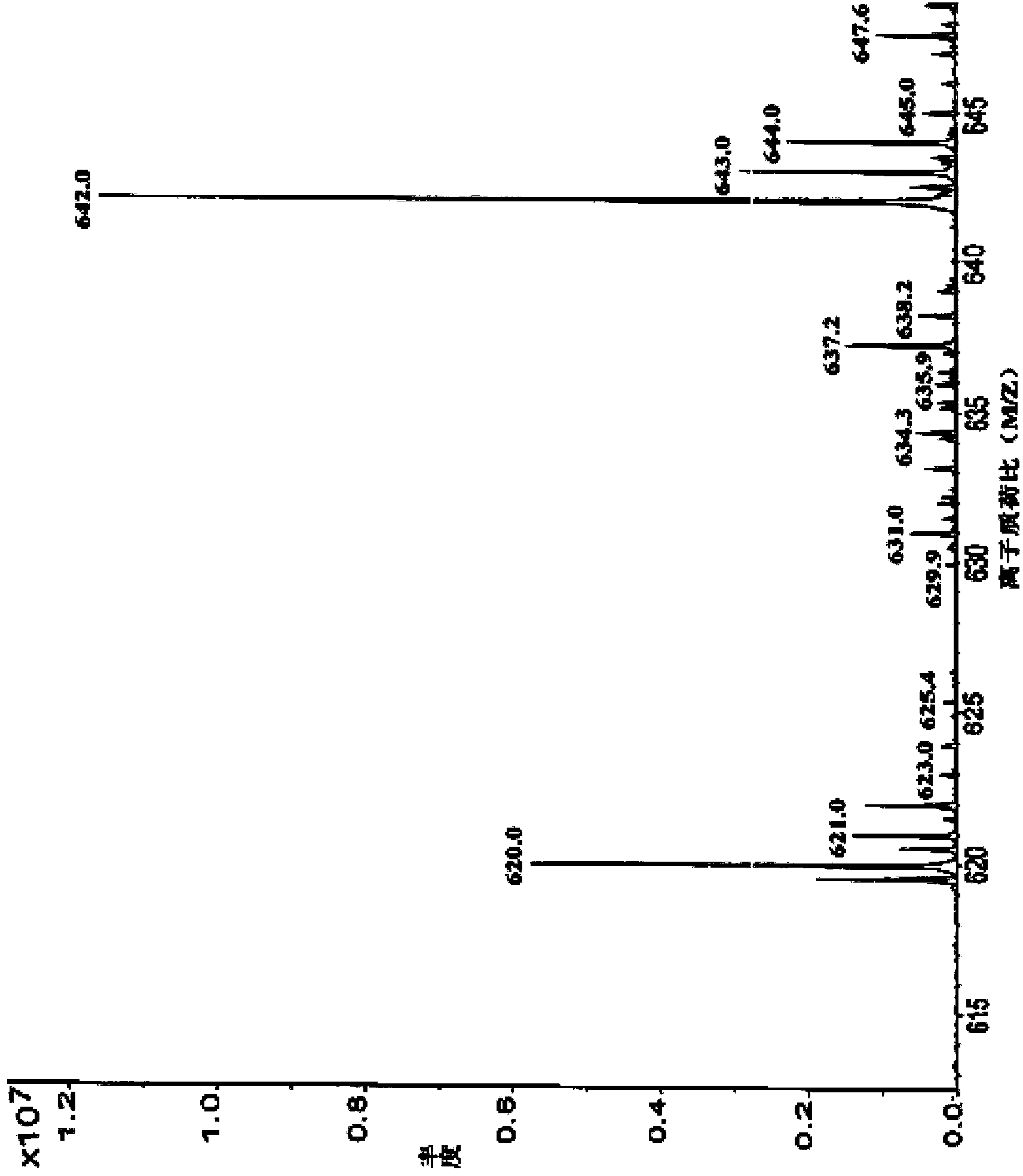
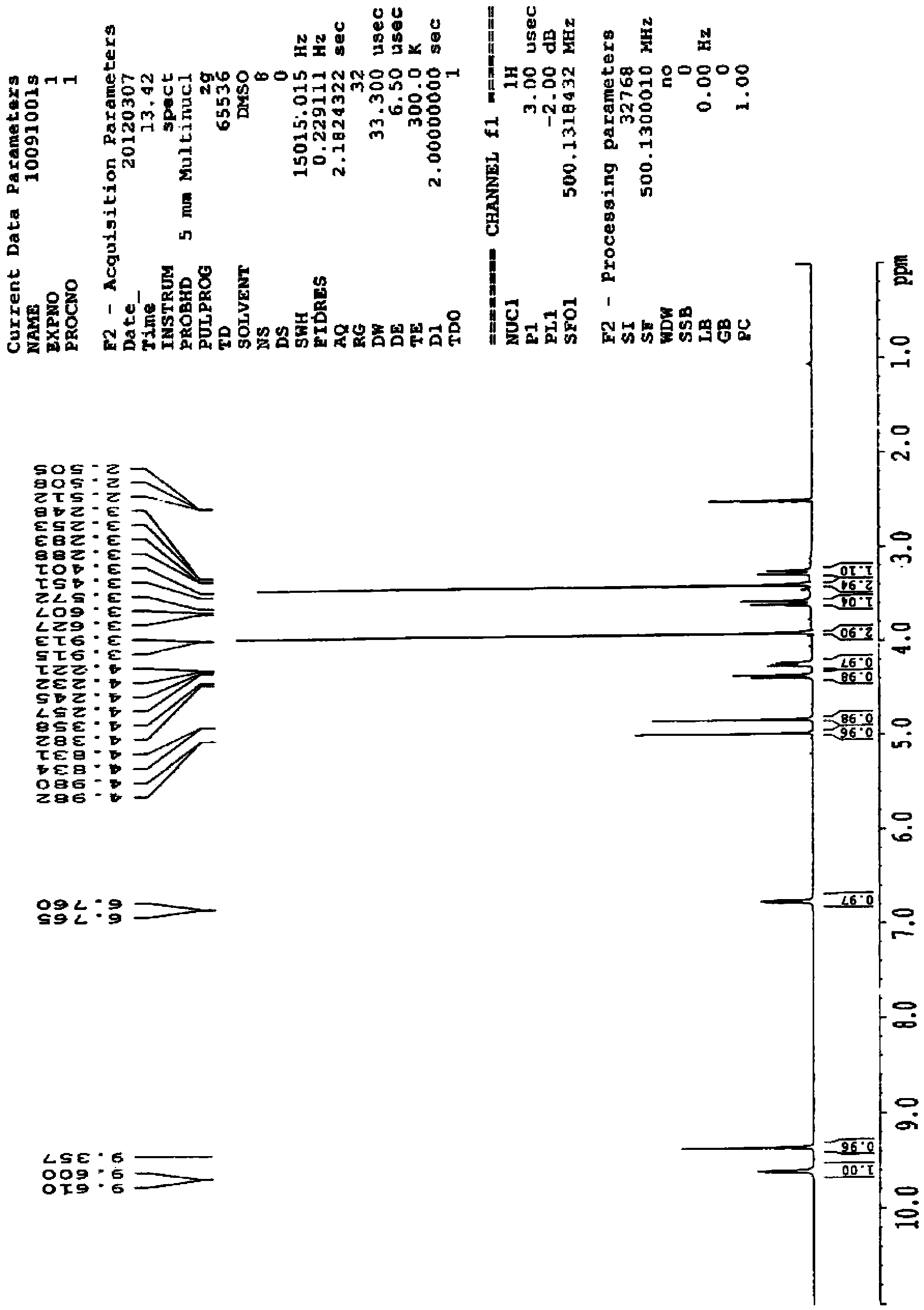
The invention discloses preparation of an impurity in cefetecol disodium and a structure confirmation method, and belongs to the pharmaceutical analysis technology field. Literature reports that a HPLC-MS method to carry out structure derivation of an impurity in a sample, but aimed to the impurity with a small content, satisfied results are difficult to obtain through the HPLC-MS method, and literature and patent reports related to the impurity are not discovered. Aimed to the above problem, separation is carried out by utilization of a preparation column, enrichment of the target impurity is carried out, further separation and purification are carried out, and finally the impurity monomer is obtained. Confirmation is carried out and the chemical structure of the impurity is obtained. The method is simple, the result is accurate, and the method can be used for impurity analysis control of cephalosporin samples.
Owner:CHENGDU UNIV
Preparation method of cefotetan disodium bulk drug
ActiveCN112552316AIncrease reaction rateHigh product yieldOrganic chemistryBulk chemical productionAcetic acidPropanoic acid
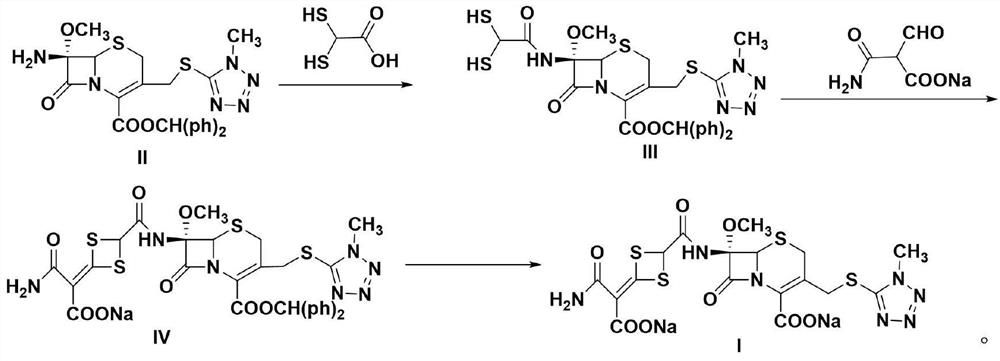
The invention discloses a preparation method of a cefotetan disodium bulk drug, which comprises the following steps: reacting 7-MAC (II) with 2, 2-dimercaptoacetic acid to prepare a compound III, reacting the compound III with 3-amino-2-formyl -3-oxo sodium propionate to prepare a compound IV, and carrying out deprotection and salification to obtain cefotetan disodium (I). The preparation method is simple and convenient, the reaction conditions are mild, and the obtained cefotetan disodium bulk drug is high in purity and suitable for industrial production.
Owner:SHANDONG LUOXIN PHARMA GRP HENGXIN PHARMA CO LTD +2
A preparation method of a high -purity injection for cephalosporin sodium
ActiveCN107141307BImprove product qualityAvoid quality risksOrganic chemistryAntibiotic YCefotetan Disodium
The invention discloses a preparation method of high-purity cefotetan disodium for injection, which belongs to the field of production and purification process of cephalosporin antibiotics. The main raw materials are 7‑MAC, acylating agent and isothiazole monosodium salt. After the acylation reaction, hydrolysis reaction and condensation reaction, the active alumina chromatography column is used to purify and remove impurities, and then after steps such as decolorization, salt formation and refining, cefotetan disodium is finally obtained; the present invention can overcome organic solvents Residue and no quality risk caused by resin crushing, better product quality and lower impurity content, and lower cost, simple operation, energy saving and environmental protection, suitable for industrial production.
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA
A method for the preparation and structure confirmation of impurities in cefotetan disodium
ActiveCN104910192BConfirmed chemical structureSimple methodOrganic chemistryChemical structureCefotetan Disodium
The invention discloses preparation of an impurity in cefetecol disodium and a structure confirmation method, and belongs to the pharmaceutical analysis technology field. Literature reports that a HPLC-MS method to carry out structure derivation of an impurity in a sample, but aimed to the impurity with a small content, satisfied results are difficult to obtain through the HPLC-MS method, and literature and patent reports related to the impurity are not discovered. Aimed to the above problem, separation is carried out by utilization of a preparation column, enrichment of the target impurity is carried out, further separation and purification are carried out, and finally the impurity monomer is obtained. Confirmation is carried out and the chemical structure of the impurity is obtained. The method is simple, the result is accurate, and the method can be used for impurity analysis control of cephalosporin samples.
Owner:CHENGDU UNIV
Cephamycin intermediate compound and preparation method thereof
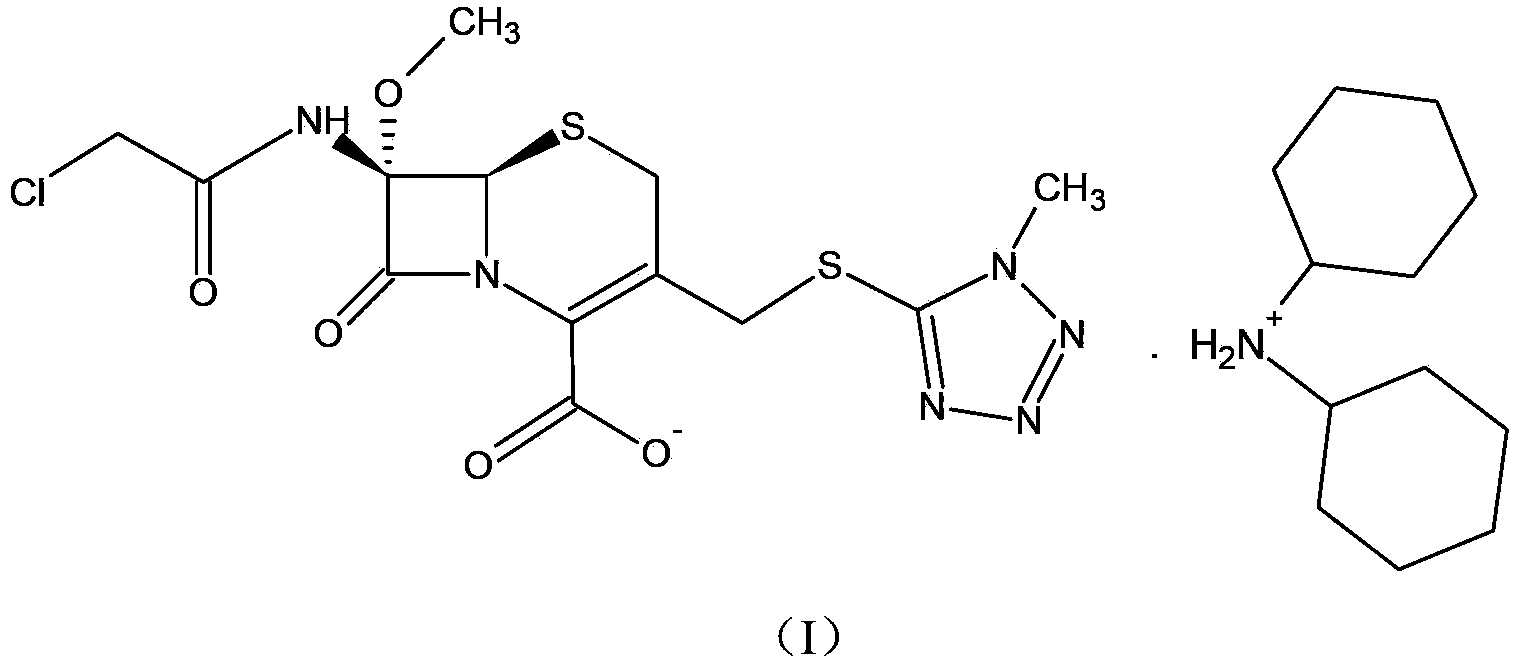
ActiveCN103193796BRaise the level of purityHigh purityOrganic compound preparationAmino compound preparationTetrazoleCEFMINOX SODIUM
The invention relates to a cephamycin intermediate compound and a preparation method thereof. The cephamycin intermediate compound has a structure of formula (I), and is prepared by directly reacting 7beta-chloroacetamide-7alpha-methoxy-3-(1-methyl-1H-tetrazole-5-thiomethyl)-3-cephem-4-carboxylic acid and dicyclohexylamine in a specific solvent to form a salt and crystallizing. The cephamycin intermediate compound has the characteristics of high purity, high stability and the like, is conductive to improving the purity of cephamycin final products such as cefminox sodium, cefmetazole sodium and cefotetan disodium, and is beneficial to large-scale production and application.
Owner:山东安弘制药有限公司
A kind of preparation method of cefotetan disodium
ActiveCN109824700BLow impurity contentImprove liquidityOrganic chemistryOrganic solventPhysical chemistry
The invention discloses a preparation method of cefotetan disodium, which belongs to the technical field of medicine and comprises the following steps: A. Adding methanol into the reactor, stirring and controlling the temperature, then adding cefotetan acid, and then adding a salt-forming agent, stirring until dissolved to obtain a mixed solution; B. Control the temperature of the reactor, add a small amount of organic solvent until the system is slightly cloudy, and grow crystals once; C. After the first crystal growth is completed, add an organic solvent, carry out secondary crystal growth, and filter to obtain solid crystals of cefotetan disodium; D. The solid crystal of cefotetan disodium is washed, atomized and dried, and vacuum-dried to obtain the finished product of cefotetan disodium. The cefotetan disodium prepared by the invention has low impurity content, good fluidity, uniform particle size distribution, stable quality, easy subpackage of subsequent powder injection preparation products, and improved drug safety; the preparation method of the invention also has the advantages of simple operation , The advantages of energy saving and environmental protection.
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA
Method for purifying cefotetan disodium
The invention suitable for the field of chemical pharmacy provides a method for purifying cefotetan disodium. The method comprises the steps as follows: providing raw materials; depositing; removing impurities; filtering; and drying. According to the method, crude cefotetan disodium is reacted with calcium salt, and cefotetan disodium isomers (II) are reacted with calcium ions to generate deposits, so that the cefotetan disodium isomers (II) are greatly reduced; and meanwhile, purified cefotetan disodium is obtained in combination with a solvent-out crystallization method, so that the production process is simplified and the production cost is reduced.
Owner:SHENZHEN CHINA RESOURCES GOSUN PHARMA CO LTD +1
A kind of preparation method of cefmetazole sodium
The invention relates to a preparation method of cefmetazole. The preparation method comprises the steps of adopting a one-pot method containing 7-ACA, 1-methyl-5-tetrazole-thione and chloroacetyl chloride, obtaining 7-DCT, then using seven-position methoxy groups of sodium methylate and tert-butyl hypochlorite, and obtaining methoxy cephalosporin mother nuclei which are shared mother nuclei. The cefmetazole can serve as four methoxy cephalosporin products, namely cefminox, cefmetazole, cephalosporin and cefotetan disodium, three steps are omitted, the processing steps are greatly shortened, cost is lowered, and the overall yield is raised to 70%.
Owner:NANJING JINHAO MEDICAL TECH CO LTD
A kind of amorphous cefotetan acid and its preparation method of cefotetan disodium and pharmaceutical composition containing the cefotetan disodium
InactiveCN103724359BImprove solubilityTroubleshoot unstable technical issuesAntibacterial agentsOrganic active ingredientsOrganic solventX-ray
Owner:SHENZHEN SALUBRIS PHARMA CO LTD
Preparation method for cefotetan disodium
The invention relates to the technical field of medicine and particularly relates to a preparation method for cefotetan disodium. In the preparation method, cefotetan acid is mixed with silica gel through a first salifying, then a first filter liquor is collected through a first filtering, the first filter liquor is mixed with EDTA and active carbon, a second filter liquor is collected through filtering, then acid-forming and crystallization are performed, and then cefotetan acid product is obtained through third filtration, collection and filter cake, as well as washing; and the cefotetan acid product is taken to be mixed with active carbon after second salifying, fourth filter liquor is collected through the fourth filtering, and then degerming and freeze drying are performed, namely the cefotetan disodium is obtained. The product prepared by using the method provided by the invention is white, is excellent in solution clarity after solution, is less in the variety of residual solvent, is low in content of relevant matters such as polymer and the like, the adopted process is simple, the raw materials are easy to access, and the method is suitable for large-scale industrial production.
Owner:CHINA MEHECO SANYANG PHARMA CO LTD
A kind of preparation method of anti-infective cephalosporin
Owner:SHANDONG LUOXIN PHARMA GRP HENGXIN PHARMA CO LTD +2
Medicinal composition of cefotetan disodium
ActiveCN101590061BNot easy to degradeAntibacterial agentsOrganic active ingredientsCefotetan DisodiumPharmaceutical Substances
The invention discloses a medicinal composition of cefotetan disodium, which comprises the cefotetan disodium, and also comprises minor ingredients; the percentage by mass of the cefotetan disodium is 50 to 99 percent; and the percentage by mass of the minor ingredients is 1 to 50 percent. The medicinal composition of the cefotetan disodium is added with the minor ingredients capable of strengthening the stability of the cefotetan disodium to inhibit the quick degradation of the cefotetan disodium when the cefotetan disodium meets water in the processes of production, storage and use and improve the stability of the cefotetan disodium, so that a high-efficiency and stable medicinal composition product of the cefotetan disodium is obtained.
Owner:SHENZHEN LIJIAN PHARM CO LTD
Preparation method of high-purity cefotetan disodium for injection
ActiveCN107141307AImprove product qualityAvoid quality risksOrganic chemistryAntibiotic YCefotetan Disodium
The invention discloses a preparation method of high-purity cefotetan disodium for injection, belonging to the process fields of production and purification of cephalosporin antibiotics. The preparation method comprises the steps of carrying out acylation reaction, hydrolysis reaction and condensation reaction on the main raw materials including 7-MAC, an acylating agent and isothiazole mono-sodium salt, carrying out purification and impurity removal by virtue of an activated alumina chromatographic column, and carrying out the steps of decoloration, salt formation, refining and the like, so as to prepare cefotetan disodium. According to the preparation method, the quality risks caused by residual solvent residues and resin-free crushing can be overcome, the product quality is relatively good, and the impurity content is relatively low; and furthermore, the preparation method is relatively low in cost, simple in operation, saving in energy, environmentally friendly and suitable for industrial production.
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA
A kind of preparation method of cephamycin intermediate
ActiveCN105017287BInhibitionHigh yieldOrganic chemistryChemical recyclingPolymer scienceCEFMINOX SODIUM
The invention relates to a preparation method of a cephamycin intermediate. The cephamycin intermediate compound has a structure of formula (IV), and is prepared by reacting 7‑ACA with dichloroacetyl chloride in a specific solvent, reacting the obtained product with MMT under the catalysis of a sulfonic acid catalyst, and crystallizing. The cephamycin intermediate of the present invention has the characteristics of high purity and good stability, and is beneficial to improving the purity of cefamycin final products such as cefminox sodium, cefmetazole sodium and cefotetan disodium, and is beneficial to large-scale production and application .
Owner:山东安弘制药有限公司
Cefotetan disodium compound as well as preparation method and medicinal composition thereof
ActiveCN102731532BExcellent acceleration stabilityProcess stabilityAntibacterial agentsOrganic active ingredientsX-rayCefotetan Disodium
Owner:永春县产品质量检验所福建省香产品质量检验中心国家燃香类产品质量监督检验中心福建
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com