A kind of amorphous cefotetan acid and its preparation method of cefotetan disodium and pharmaceutical composition containing the cefotetan disodium
A cefotetan acid, amorphous technology, applied in the field of drug invention, to achieve the effect of improving the dissolution effect, improving the bioavailability, and simplifying the filtration and drying process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] The preparation of embodiment 1 cefotetan acid
[0050] (1) Amidation reaction
[0051] Dissolve 100g of 7-MAC in 1500ml of dichloromethane, stir to dissolve 7-MAC, cool down to below 5°C, add 40g of N,N-methylaniline, add 10 minutes, stir for 20 minutes, add 55g of bromoacetyl bromide , 30 minutes to complete the addition, heat preservation and stirring until the reaction is complete (7-MAC residue is less than 1.5%), add 2% sulfuric acid solution to the reaction solution, stir, stand still for phase separation, collect the organic phase, and obtain the reaction solution A.
[0052] (2) Dediphenyl ester reaction
[0053] Take 100g of aluminum trichloride and add it into 300ml of dichloromethane, stir, cool down to below 5°C, then add 120ml of anisole, stir to obtain reaction solution B1.
[0054] The temperature of the reaction solution A was lowered to below 0°C, and the reaction solution B1 was added to react for 1 hour to obtain the reaction solution B2.
[0055]...
Embodiment 2
[0058] Example 2 Preparation of Cefotetan Acid Amorphous Crystals
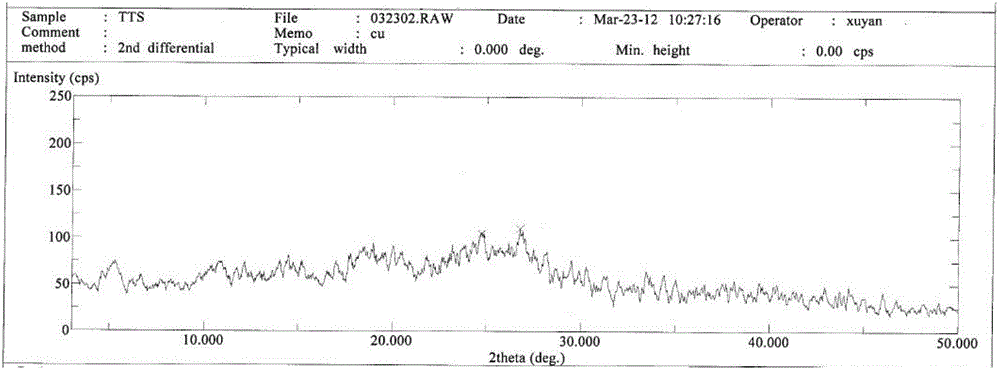
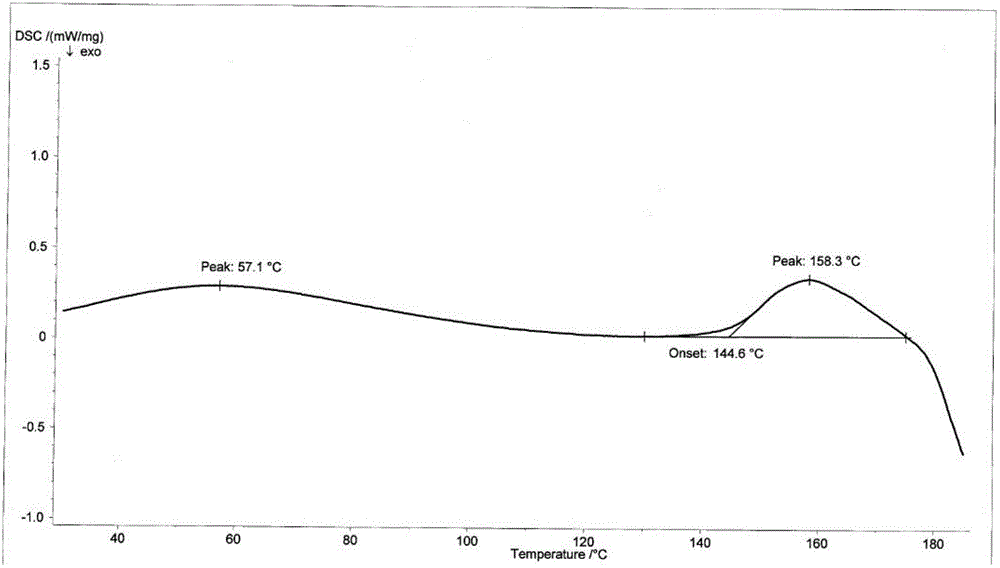
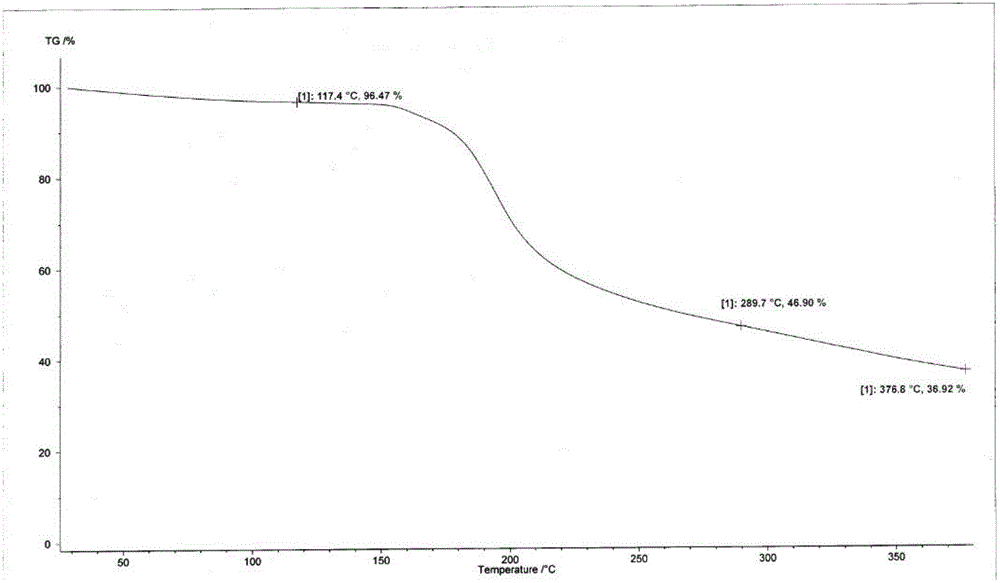
[0059] Suspend the crude cefotetan acid obtained in Example 1 in water, add hydrochloric acid to adjust the pH to 1.5-2.5, then add 2000ml butanone, stir for half an hour, and filter. Concentrate the butanone phase under reduced pressure below 40°C until 1 / 3 to 1 / 4 of the butanone liquid remains, add 1000ml of ethanol, cool down to below 0°C to grow crystals for 3 hours, filter, wash the filter cake with 300ml of ethanol, and keep it below 50°C Vacuum-dried until the water content was less than 2.0%, discharged and weighed to obtain 723 g of amorphous cefotetan acid with a purity of 98.7%. The X-ray diffraction spectrum of gained amorphous crystalline product, differential scanning calorimetry (DSC) collection of illustrative plates and thermogravimetric analysis (TG) collection of illustrative plates are respectively as follows figure 1 , figure 2 , image 3 shown.
[0060] The obtained amorphous cefotet...
Embodiment 3
[0061] Example 3 Preparation of Cefotetan Disodium
[0062] Take 700 g of cefotetan acid in Example 2, add 7000 ml of water for injection, start stirring, lower the temperature to below 10°C, and slowly add solid sodium bicarbonate until the solid dissolves. Add gac 70g, stir 40 minutes, through decarbonization filtration, sterilizing filtration, carry out lyophilization, to moisture is less than 2.0%, discharging, weigh, obtain cefotetan disodium 650g, purity 98.4%, yield 92.8%. m / e 620.3[M+H] + , 1 H-NMR(500MHz,DMSO)δ(ppm):9.605(d=5.0,1H),9.357(s,1H),6.762(m,1H),4.982(s,0.5H)4.980(s,0.5H) ,4.831(s,0.5H),4.370(d=12.5,0.5H),3.915(s,1.5H),3.585(d=17.5,1H),3.401(s,3H),3.250(d=17.5,1H ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com