Preparation method of cefotetan disodium
A technology for cefotetan disodium and cefotetan acid, which is applied in the field of preparation of cefotetan disodium, can solve the problems of low product purity, poor appearance quality, complicated process and the like, and achieves high product yield, stable medicinal properties, simple craftsmanship
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
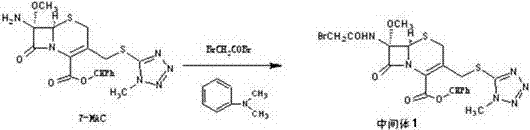
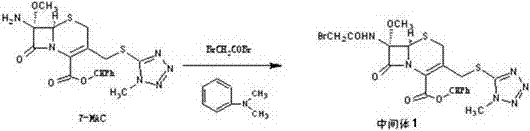
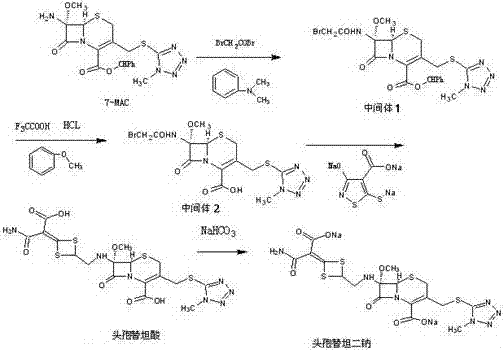
[0030] The invention discloses a preparation method of cefotetan disodium, using 7-amino-7-methoxy-3-1-methyl-1H-5-tetrazolyl)thiomethyl-3-cephem- 4-diphenylmethyl carboxylate (7-MAC) was used as the starting material, and bromoacetyl bromide was subjected to amidation reaction at low temperature to obtain 7-bromoacetamide-7-methoxy-3-(1-methyl Base-1H-5-tetrazolyl)thiomethyl-3-cephem-4-carboxylate diphenylmethyl ester (intermediate 1), the product of this step is directly subjected to the next step reaction without isolation to obtain the product 7-bromoethyl Amide-7-methoxy-3-(1-methyl-1H-5-tetrazolyl)thiomethyl-3-cephem-4-carboxylic acid (intermediate 2), and finally with 4-carboxy-3 -Hydroxy-5-mercaptoisothiazole trisodium (CHMT) was reacted to obtain cefotetan acid, and the acid was converted to sodium salt, and then sterile filtered, freeze-dried and pulverized to obtain cefotetan disodium sterile powder.
[0031] The reaction formula is as follows:
[0032]
[0033...
Embodiment 1
[0049] Add 60~80L ethyl acetate, 3.5~4kg 7-amino-7-methoxy-3-(1-methyl-1H-5-tetrazolyl)thiomethyl-3-cephem- 4-Diphenylmethyl carboxylate, stirred to dissolve, cooled in ice-water bath to -5~0℃, then added 900~1000g N,N-dimethylaniline, then added dropwise 1.8~2.5kg bromoacetyl bromide,- Under constant temperature conditions of 5-0°C, stir at a stirring rate of 50-80r / min to carry out amidation reaction for 15-30min; then add ice water and ethyl acetate mixed at a volume ratio of 1:3-5 to the reaction liquid The ester was shaken to separate the organic layer, and the organic layer was washed with 0.2-0.5 mol / L hydrochloric acid solution for more than 3 times, then dried with sodium sulfate, filtered, and the filtrate was distilled under reduced pressure at 50°C to remove the organic solvent to obtain the first intermediate 7-Bromoacetamide-7-methoxy-3-(1-methyl-1H-5-tetrazolyl)thiomethyl-3-cephem-4-carboxylic acid benzhydryl ester;
[0050] Add 40-50L of dichloromethane and 4-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com