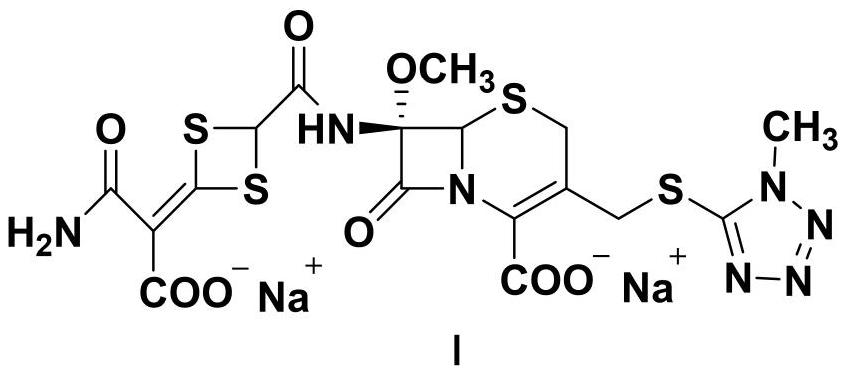
Preparation method of cefotetan disodium bulk drug
A technology of cefotetan disodium and raw materials, which is applied in the field of preparation of cefotetan disodium raw materials, can solve the problems of long cycle, low product yield and high cost, achieve mild reaction conditions, increase the total yield and Purity, effect of increasing reaction rate and product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
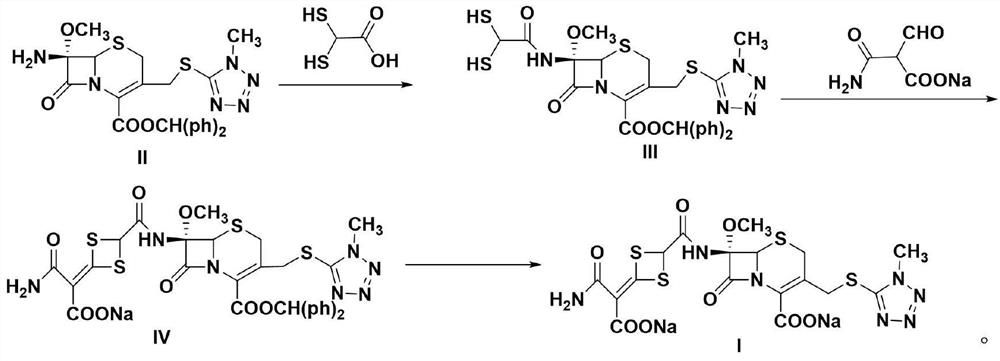
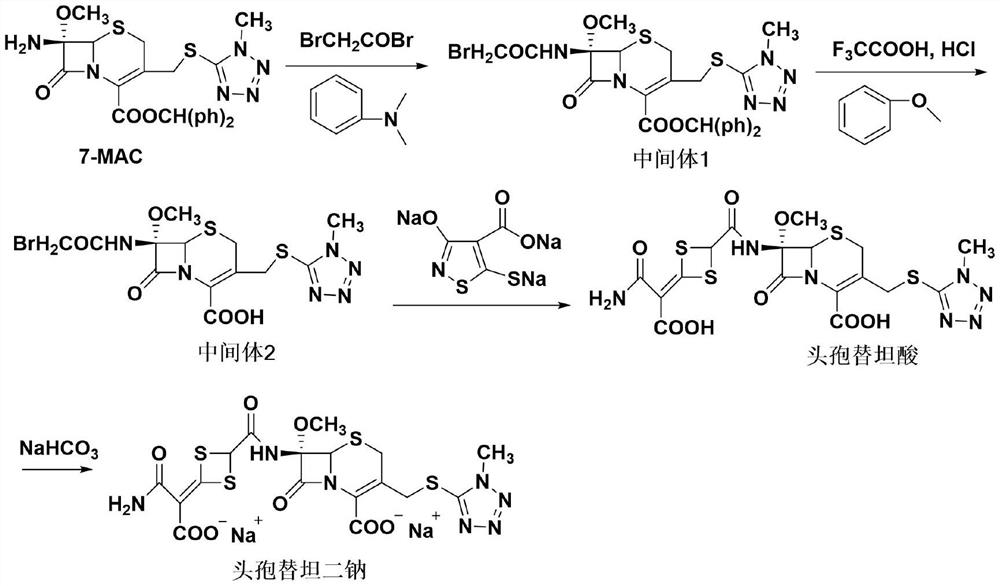
[0027] Preparation of Compound III
[0028] Add 2,2-dimercaptoacetic acid (2.74g, 0.022mol, molecular weight 124.17) and 15mL ethyl acetate solution in the constant pressure funnel, add compound II 7-MAC (10.5g, 0.02mol, molecular weight 524.61) in the reactor and 60mL ethyl acetate, add the condensing agent CDI (5.19g, 0.032mol, molecular weight 162.15) in batches, stir to dissolve, slowly add the solution in the constant pressure funnel dropwise to the reactor, the reaction temperature is 40°C, the reaction time is 10h, TLC Follow the reaction until the complete disappearance of 2,2-dimercaptoacetic acid, extract the organic phase, dry, filter and concentrate to obtain 12.44g (0.0197mol, molecular weight: 630.77) of compound III, with a yield of 98.6%, HPLC purity of 99.7%, and a maximum of 0.02%.
Embodiment 2
[0030] Preparation of Compound III
[0031] Add 2,2-dimercaptoacetic acid (2.74g, 0.022mol, molecular weight 124.17) and 15mL ethyl acetate solution in the constant pressure funnel, add compound II 7-MAC (10.5g, 0.02mol, molecular weight 524.61) in the reactor and 60mL ethyl acetate, add the condensing agent DCC (6.59g, 0.032mol, molecular weight 206) in batches, stir to dissolve, slowly add the solution in the constant pressure funnel dropwise to the reactor, the reaction temperature is 40°C, the reaction time is 10h, TLC Follow the reaction until the complete disappearance of 2,2-dimercaptoacetic acid, extract the organic phase, dry, filter and concentrate to obtain 12.35 g (0.0195 mol, molecular weight: 630.77) of compound III, the yield is 97.9%, the HPLC purity is 99.6%, and the maximum heterogeneity is 0.02%.
Embodiment 3
[0033] Preparation of Compound III
[0034] Add 2,2-dimercaptoacetic acid (2.74g, 0.022mol, molecular weight 124.17) and 15mL ethyl acetate solution in the constant pressure funnel, add compound II 7-MAC (10.5g, 0.02mol, molecular weight 524.61) in the reactor and 60mL of ethyl acetate, add the condensing agent DIC (4.04g, 0.032mol, molecular weight 126.20) in batches, stir to dissolve, slowly add the solution in the constant pressure funnel into the reactor, the reaction temperature is 40°C, the reaction time is 10h, TLC Follow the reaction until the complete disappearance of 2,2-dimercaptoacetic acid, extract the organic phase, dry, filter and concentrate to obtain 12.11 g (0.0192 mol, molecular weight: 630.77) of compound III, with a yield of 96.6%, HPLC purity of 99.7%, and a maximum of 0.03%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com