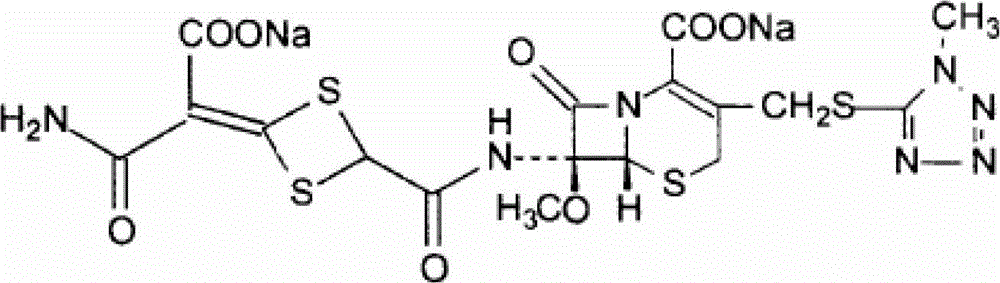
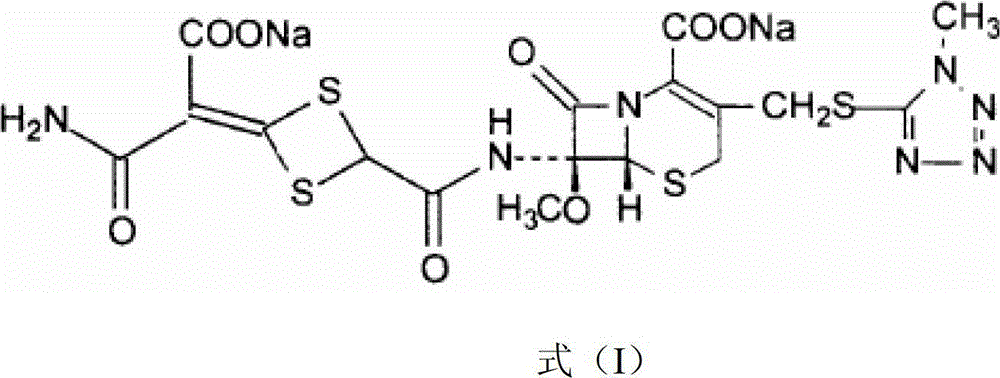
Cefotetan disodium compound as well as preparation method and medicinal composition thereof
A technology of cefotetan disodium and compound, applied in the directions of organic chemistry, antibacterial drugs, pharmaceutical formulations, etc., can solve the problems of difficulty in production, storage and use, instability of cefotetan disodium, unqualified products, etc. Stable product quality, avoiding potential safety hazards, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] [Example 1] Preparation of Cefotetan Disodium Compound
[0068] 1) dissolving cefotetan disodium in a mixed solvent of tetrahydrofuran and methanol to obtain a tetrahydrofuran / methanol solution of cefotetan disodium;
[0069] 2) Add chloroform dropwise to the tetrahydrofuran / methanol solution of cefotetan disodium obtained in step 1) under an ultrasonic field until crystals are precipitated;
[0070] 3) Turn off the ultrasonic field, let stand, filter, wash the filter cake with methanol, and dry to obtain the cefotetan disodium compound.
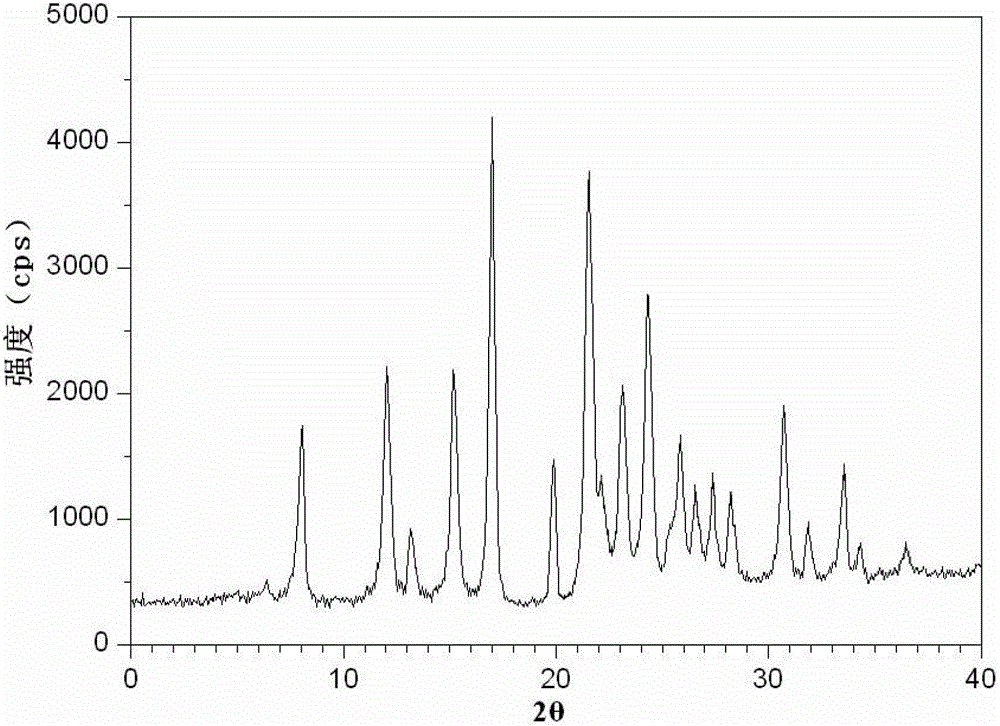
[0071] The resulting cefotetan disodium compound uses Cu-Kα rays to measure the characteristic peaks in the X-ray powder diffraction pattern obtained at 2θ of 8.0°, 12.1°, 15.4°, 17.0°, 19.8°, 21.6°, 23.0°, 24.3°, 25.7°, 27.4°, 30.7° and 33.5° are displayed.
[0072] Below is embodiment 2-9, and preparation method is with embodiment 1, and its concrete process parameter is shown in Table 1:
[0073] Table 1
[0074]
[0075] Th...
preparation Embodiment 1
[0076] [Preparation Example 1] Cefotetan Disodium Freeze-Dried Powder Injection
[0077]
[0078] Preparation Process:
[0079] (1) Weigh the cefotetan disodium compound prepared in Example 1 of the prescription amount and add it to 80% of the total volume of water for injection, stir to obtain a solution;
[0080] (2) Measure the pH of the solution (the range is 4.5-5.5), and adjust the pH with 0.1mol / L dilute hydrochloric acid if necessary;
[0081] (3) Add water for injection to 100%;
[0082] (4) Add 0.1% activated carbon, stir, let stand for 20 minutes, filter and decarbonize, and filter with a 0.22μm filter membrane;
[0083] (5) Intermediate detection;
[0084] (6) Fill the above solution at a volume of 4ml / bottle (specification: 1g), stopper halfway, freeze-dry, and stopper and cap.
[0085] (7) Fully inspect the above-mentioned freeze-dried products, and obtain them after passing the test.
preparation Embodiment 2
[0086] [Preparation Example 2] Cefotetan Disodium Freeze-Dried Powder Injection
[0087]
[0088] Preparation process: same as Example 1, except that the cefotetan disodium compound used is prepared in Example 2, and filled in step (6) with a volume of 8ml / bottle (specification: 2g).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Bronsted acidity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com