Cefotetan disodium for injection, and preparation method thereof
A technology of cefotetan disodium and cefotetan, which is applied in the field of cefotetan disodium for injection and its preparation, and can solve the problems of inability to obtain impurity content, potential safety hazards for patients, and high impurity content, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
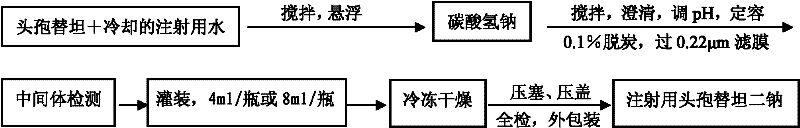
[0082] prescription:
[0083] Preparation Process:
[0084] (1) The cefotetan that takes prescription quantity is added in the cooling water for injection (0~5 ℃) of 80% of total volume, stir, make it become suspension;
[0085] (2) Take by weighing the sodium bicarbonate of recipe quantity, slowly join in above-mentioned suspension, fully stir, make solution clarification;
[0086] (3) Measure the pH of the solution (in the range of 4.5 to 5.5), and adjust the pH with 0.1mol / L dilute hydrochloric acid if necessary;
[0087] (4) Add cooled water for injection (0-5°C) to 100%;
[0088] (5) Add 0.1% activated carbon, stir, let stand for 20 minutes, filter for decarbonization, and filter with a 0.22 μm filter membrane;
[0089] (6) Intermediate detection;
[0090] (7) Fill the above solution in a volume of 4ml / bottle (specification: 1g), stopper halfway, freeze-dry, and stopper and cap.
[0091] (8) All the above-mentioned freeze-dried products are inspected, and cefotetan...
Embodiment 2
[0093] prescription:
[0094] Preparation process: the same as in Example 1, except that it is filled in step (7) at a capacity of 8ml / branch (specification: 2g).
Embodiment 3
[0096] prescription:
[0097] Preparation Process:
[0098](1) The cefotetan that takes prescription quantity joins in the water for injection of 0 ℃ of 80% of total volume, stirs, and makes it become suspension;
[0099] (2) Take by weighing the sodium bicarbonate of recipe quantity, slowly join in above-mentioned suspension, fully stir, make solution clarification;
[0100] (3) Measure the pH of the solution (in the range of 4.5 to 5.5), and adjust the pH with 0.1mol / L dilute hydrochloric acid if necessary;
[0101] (4) Add water for injection at 0°C to 100%;
[0102] (5) Add 0.1% activated carbon, stir, let stand for 20 minutes, filter for decarbonization, and filter with a 0.22 μm filter membrane;
[0103] (6) Intermediate detection;
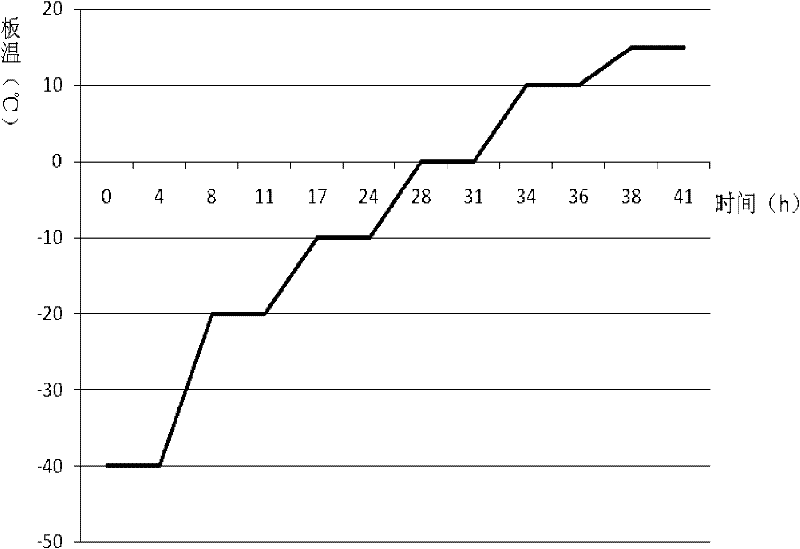
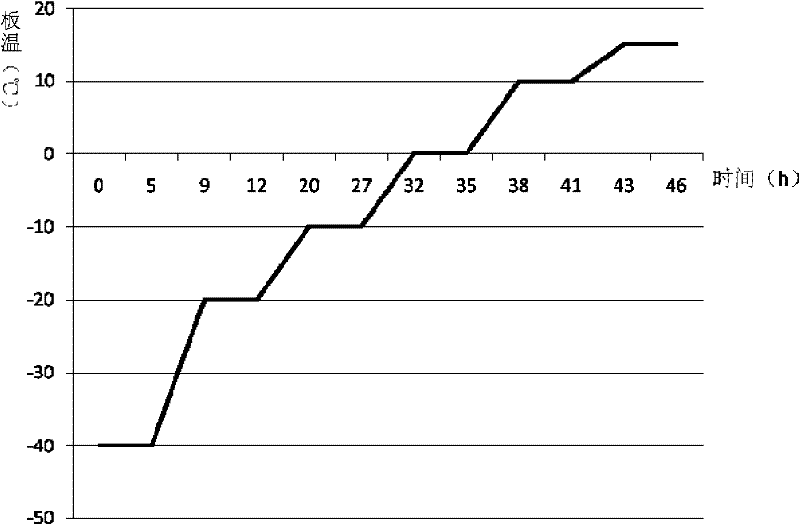
[0104] (7) Fill the above solution according to the amount of 4ml / cartridge (specification: 1g), half-stopper, freeze-dry, and stopper and cap; wherein the freeze-drying method is: start the freeze-drying machine, after pre-freezing at...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com