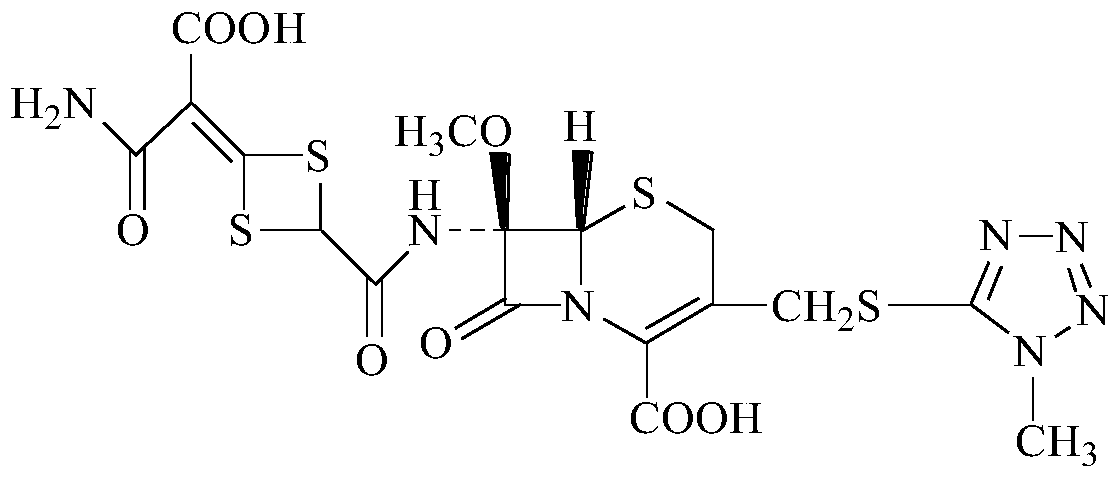
A kind of preparation method of cefotetan disodium
A technology for cefotetan disodium and cefotetan acid, which is applied in the field of cefotetan disodium, can solve the problems of high impurity content, unstable quality and poor fluidity, and achieves low impurity content, good fluidity and high yield. rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] A preparation method of Cefotetan Disodium, comprising the steps of:
[0030] A. Add methanol to the reactor, stir and control the temperature at 0-5°C, then add cefotetan acid, then add the salt-forming agent, stir until dissolved, and obtain a mixed solution; the volumetric dosage of methanol is the same as that of cefotetan acid The weight ratio of the feed is 13-17:1mL / g; the salt-forming agent is sodium isooctanoate or anhydrous sodium acetate, and the molar ratio of the salt-forming agent to cefotetan acid is 2-2.08:1.
[0031] B. Control the temperature at 3-7°C, first add a small amount of organic solvent to the mixed solution until the system is slightly muddy, and then perform a crystal growth, the crystal growth time is 60-90 minutes; the organic solvent is absolute ethanol or isobutanol, The adding method of the organic solvent is dropwise, and the dropping rate of the organic solvent per 30 g of cefotetan acid is 4-8 mL / min.
[0032] C. Add an organic solv...
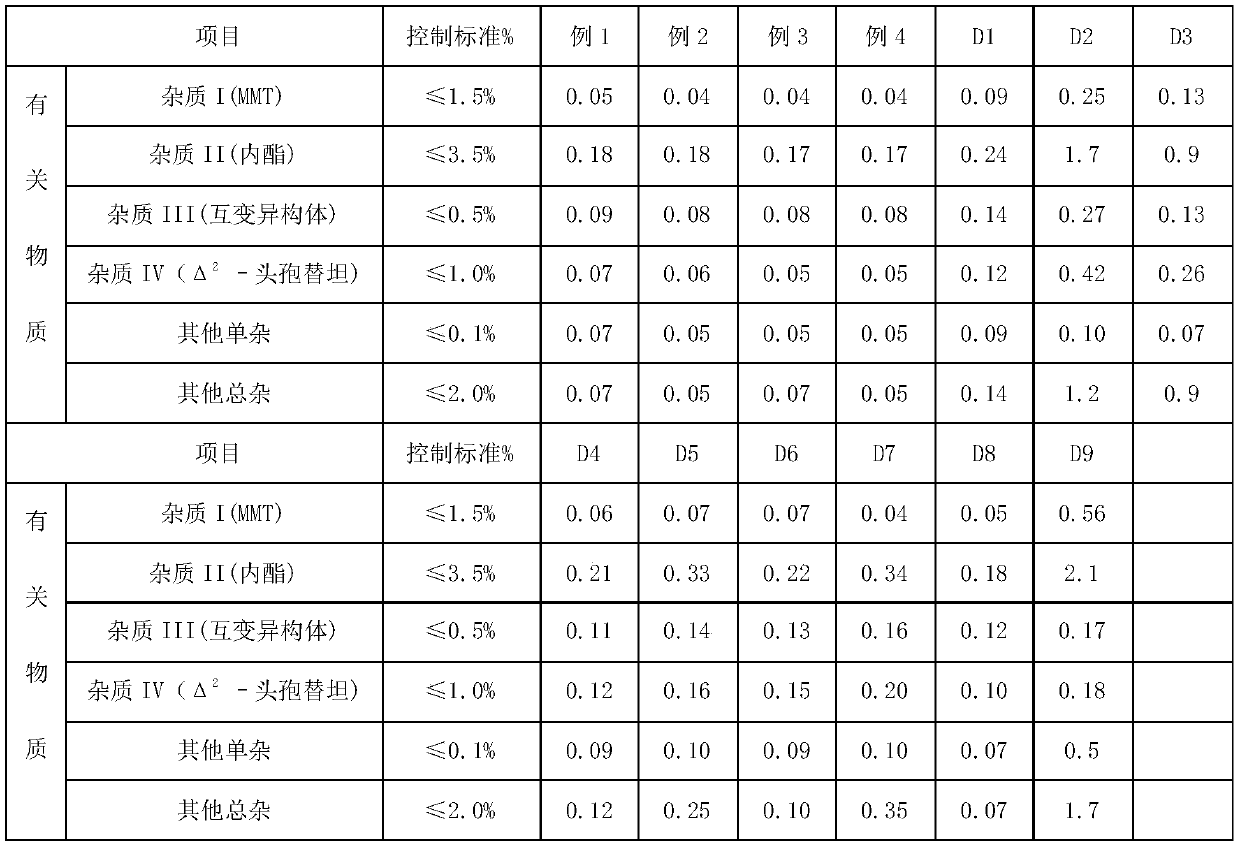
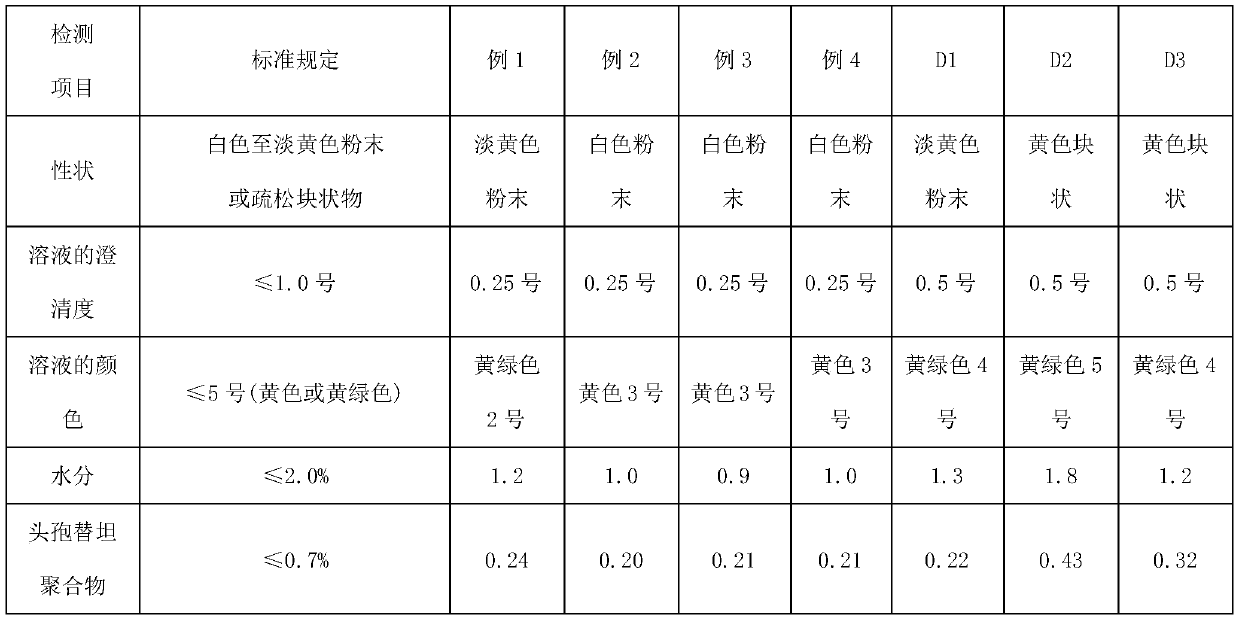
Embodiment 1
[0035] A preparation method of Cefotetan Disodium, comprising the steps of:
[0036] A. Add 390mL of methanol to the reaction bottle, start stirring and control the temperature at 5°C, add 30g of cefotetan acid, add 9.3g of anhydrous sodium acetate, stir to dissolve completely, and obtain a mixed solution.
[0037] B. Control the temperature of the reaction system at 7°C, add ethanol dropwise to the mixed solution at a flow rate of 4 mL / min until slightly turbid, stop feeding, and grow crystals for 60 min.
[0038] C. Add ethanol at 12mL / min after the crystal growth in step B, add 900mL of ethanol in steps B and C, stop feeding, control the temperature at 3°C for crystal growth for 30 minutes, and filter to obtain cefotetan disodium solid crystals.
[0039] D. Wash the cefotetan disodium solid crystal once with 30mL absolute ethanol, wash once with the mixture of 30mL ethanol and 60mL acetone, atomize 3mL methanol through a methanol atomization device, and use sterile nitrog...
Embodiment 2
[0041] A preparation method of Cefotetan Disodium, comprising the steps of:
[0042] A. Add 510mL of methanol to the reaction bottle, start stirring and control the temperature at 0°C, add 30g of cefotetan acid, add 19.5g of sodium isooctanoate, stir to dissolve completely, and obtain a mixed solution.
[0043] B. Control the temperature of the reactor at 3°C, add ethanol dropwise to the mixed solution at a flow rate of 8 mL / min until slightly turbid, stop feeding, and grow crystals for 90 min.
[0044]C. Add ethanol at 16mL / min after the crystal growth in step B, add 1200mL ethanol in steps B and C, stop feeding, control the temperature at 3°C for crystal growth for 60min, and filter to obtain cefotetan disodium solid crystals.
[0045] D. Wash the cefotetan disodium solid crystal once with 30mL of absolute ethanol, wash once with 45mL of ethanol and 60mL of acetone mixture, atomize 9mL of methanol through a methanol atomization device, and use sterile nitrogen to drive the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com