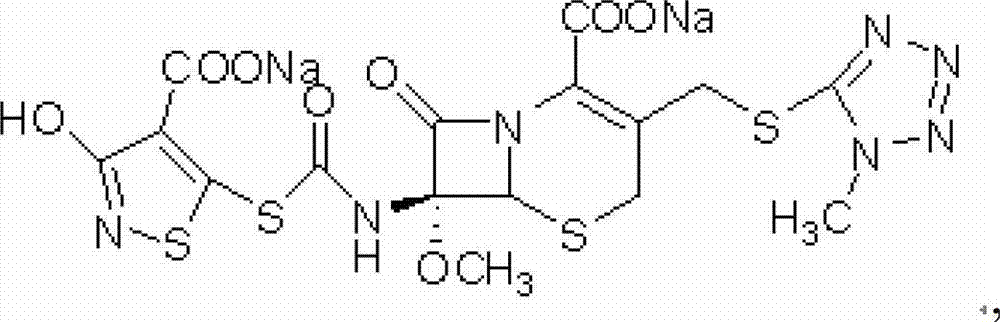
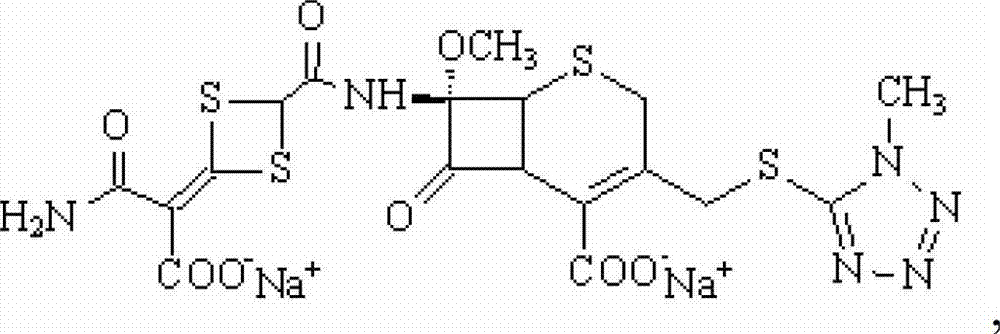
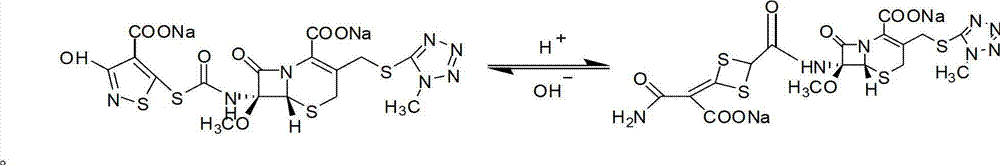
Method for purifying cefotetan disodium
A technology of cefotetan disodium and a purification method, which is applied in the field of chemical pharmacy, can solve the problems of high isomer content of cefotetan disodium, achieve the effects of simplifying the production process and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] At 0-5°C, add 50g of cefotetan disodium crude product (the content of isomers detected by HPLC is (3.55%)) into 180mL of water to dissolve completely, and add 0.38g of anhydrous calcium chloride ( The molar ratio to the isomer is 1.2:1) dissolved in 5mL of water, and at 0-5°C, add the calcium chloride aqueous solution to the cefotetan disodium aqueous solution within 30 minutes. Stir for 60 minutes, filter, wash the precipitate with 10mL of water, and combine the filtrates (the content of isomers detected by HPLC is 0.01%);
[0040] At 0-5°C, add the filtrate dropwise to 2000mL of absolute ethanol for crystallization within 60 minutes. After the dropwise addition, grow the crystals at the same temperature for 60 minutes, filter, wash with acetone 200mLx3, drain, and transfer the product to Put it into the desiccator, and use the method of gradient temperature to dry the product, that is, under the vacuum pressure greater than 0.98MPa, dry at a drying temperature of 25°C...
Embodiment 2
[0041] Example 2Ca 2+ Effect of ion dosage on isomers and yields.
[0042] According to the preparation method of embodiment 1, adopt different Ca 2+ Purification of Cefotetan Disodium Crude by Ion Dosage In addition to Ca 2+ Except ion consumption, other reactant and content are identical with embodiment 1, and experimental result is as shown in table 1 below:
[0043] Table 1: Different Ca 2+ Experimental results of ion dosage:
[0044]
[0045] From the above results, it can be seen that: Ca 2+ The ion can effectively remove the isomers of cefotetan disodium, and its dosage has a great influence on the content of the isomers of cefotetan disodium and the yield of the product. If the dosage is small, the isomers cannot be completely removed. A part of cefotetan disodium will also precipitate out, which has a greater impact on the yield. Moreover, the isomer content in the crude product of cefotetan disodium has no clear impact on product quality, as long as the corre...
Embodiment 3
[0046] Example 3 Effect of Gradient Heating on Isomer Content.
[0047] According to the preparation method of Example 1, different gradient heating curves are adopted, wherein except that the heating curve is different during drying, other reactants and contents are the same as in Example 1, and the experimental results are shown in Table 2 below:
[0048] Table 2: Experimental results of different heating schemes:
[0049]
[0050]
[0051] From the above results, it can be seen that the heating curves made from different heating schemes are different. To achieve qualified moisture, the drying time is quite different, and the isomer content difference after drying is also relatively large. Comprehensive the above schemes , heating curve 4 is more suitable for process requirements.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap