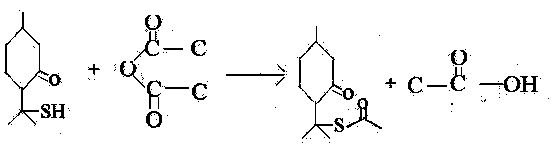
Method for synthesizing cis-and trans-menthone-8-thioacetate
A synthetic method and technology of menthone, which is applied in the field of synthesis of cis and trans-menthone-8-thiol acetate, can solve the problems of high price and low conversion rate, and achieve simple operation, mild reaction and few isomers Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] A kind of synthetic method of cis and trans-menthone-8-thiol acetate, carries out following steps successively:
[0014] 1), Acetylation: Add 120-240g acetic anhydride, 0.04-0.4g anhydrous sodium acetate, 40g thiomenthone into a 250ml three-necked flask, heat up to 100-120°C, and distill out the generated Acetic acid until the 8-thiomenthone content in the reaction system is less than 1% according to GC analysis, under vacuum conditions, the excess acetic anhydride after the reaction is distilled out, and the reaction time is 30 hours.
[0015] 2) Neutralization and washing: add the remaining liquid in the reaction bottle to a 500ml separating funnel, then add 300ml of 10% sodium carbonate aqueous solution to shake fully, let it stand, separate the alkaline water, and wash it once with clean water to obtain 49.6g of crude material.
[0016] 3) Distillation: Add 0.25g of sodium chloride to the crude material during distillation, and gradually increase the internal temper...
Embodiment 2
[0018] A kind of synthetic method of cis and trans-menthone-8-thiol acetate, carries out following steps successively:
[0019] 1) Acetylation: Add 300-600g of acetic anhydride, 0.1-1.0g of anhydrous sodium acetate, and 100g of thiomenthone into a 1000ml three-necked flask, heat up to 100-120°C, and distill out the generated during the reaction under vacuum conditions Acetic acid, until the 8-thiomenthone content in the reaction system is less than 1% according to GC analysis, under vacuum conditions, the excess acetic anhydride after the reaction is distilled out, and the reaction time is 35 hours.
[0020] 2) Neutralization and washing: Add the remaining liquid in the reaction bottle to a 1000ml separating funnel, then add 750ml of 10% sodium carbonate aqueous solution to shake fully, let it stand, separate the alkaline water, and wash it once with clean water to obtain 125.3g of crude material.
[0021] 3) Distillation: Add 0.625g of sodium chloride to the crude material du...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com