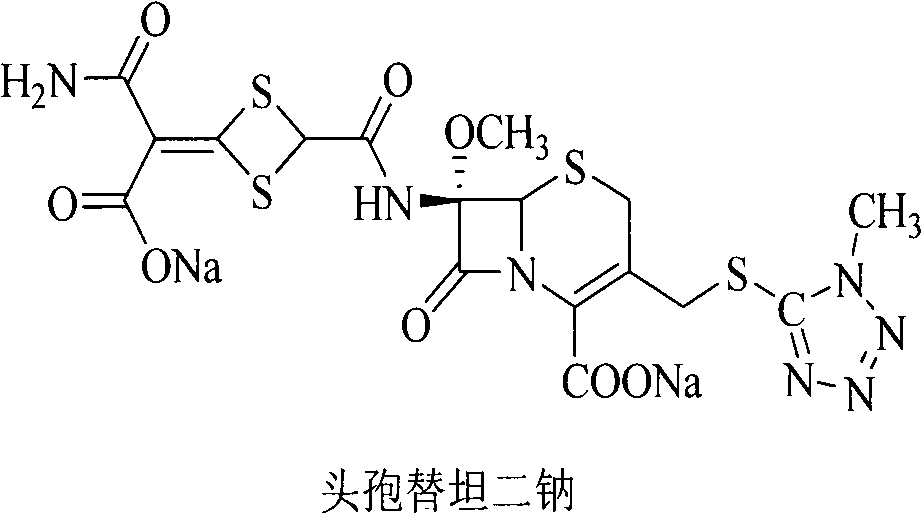
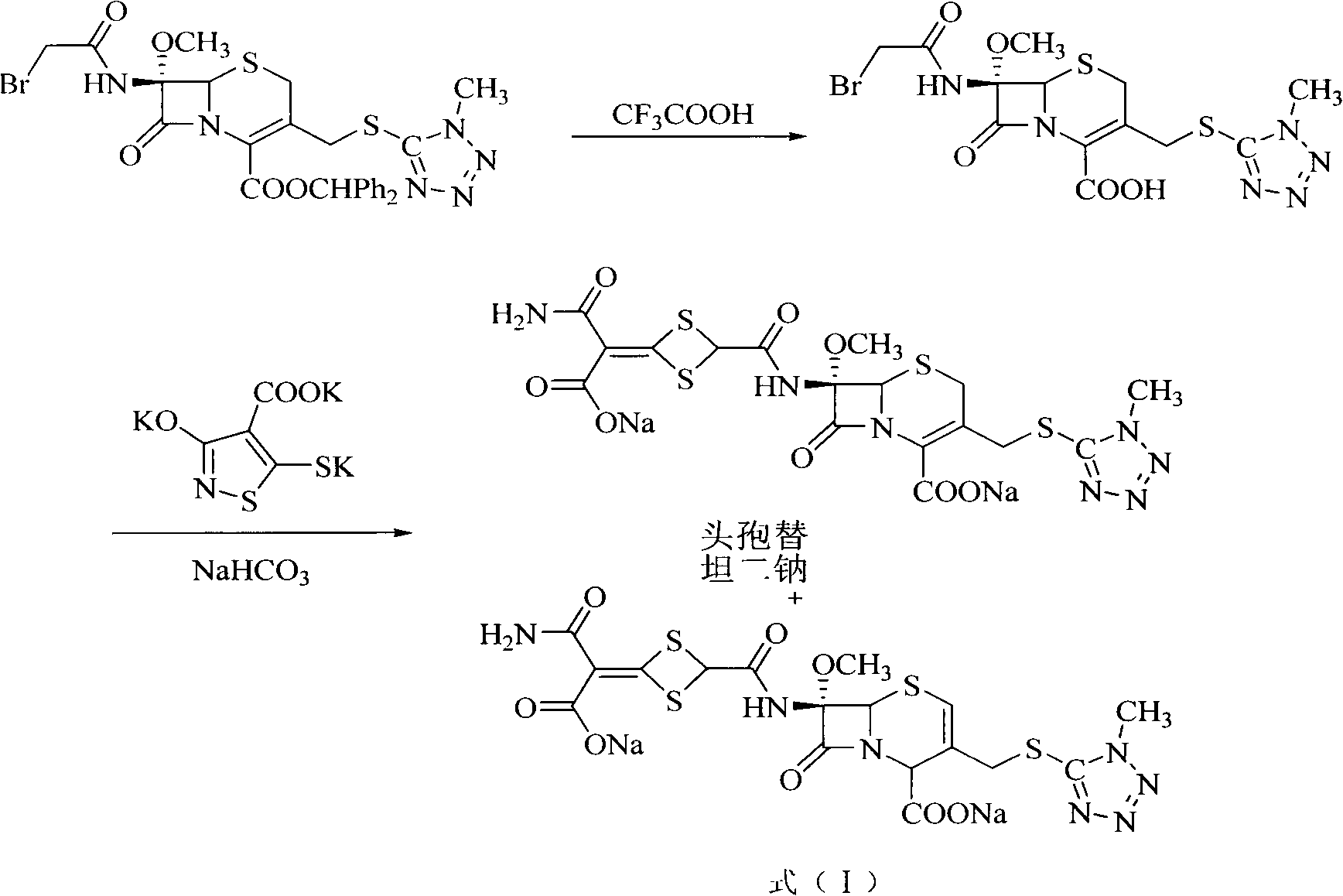
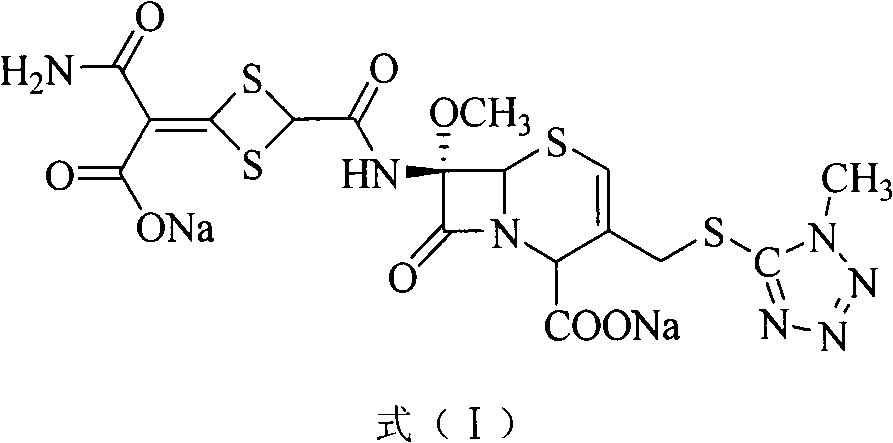
Method for removing impurity delta<2>-isomer from cefotetan disodium
A technology for cefotetan disodium and impurities is applied in the field of removing impurity Δ2-isomer in cefotetan disodium, which can solve the problems of short process route, affecting the product quality and drug safety of cefotetan disodium, and shortening the reaction time. time, simplified post-processing, high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Weigh 10.0g of crude cefotetan disodium, 0.5g of cuprous hydride, and 1.3g of tributyltin hydride into 200mL of acetonitrile, stir and mix evenly under nitrogen protection, keep the reaction at 40°C for 1 hour, and pour into 5% dilute hydrochloric acid , 1200ml of ethyl acetate extracts the aqueous phase three times, the ethyl acetate phases are combined, the ethyl acetate phase is washed with 250ml of water, dried over anhydrous sodium sulfate, filtered, the ethyl acetate is concentrated under reduced pressure, treated with sodium bicarbonate and filtered to obtain cefotetan II Sodium 9.4g, yield 94%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com