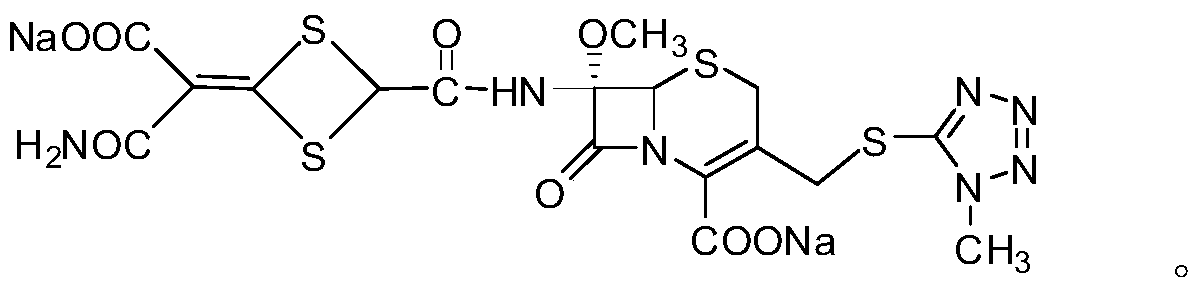
A method for the preparation and structure confirmation of impurities in cefotetan disodium
A cefotetan disodium and impurity technology, applied in the field of drug analysis, can solve problems such as limitations, many clinical side effects, and impact on safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
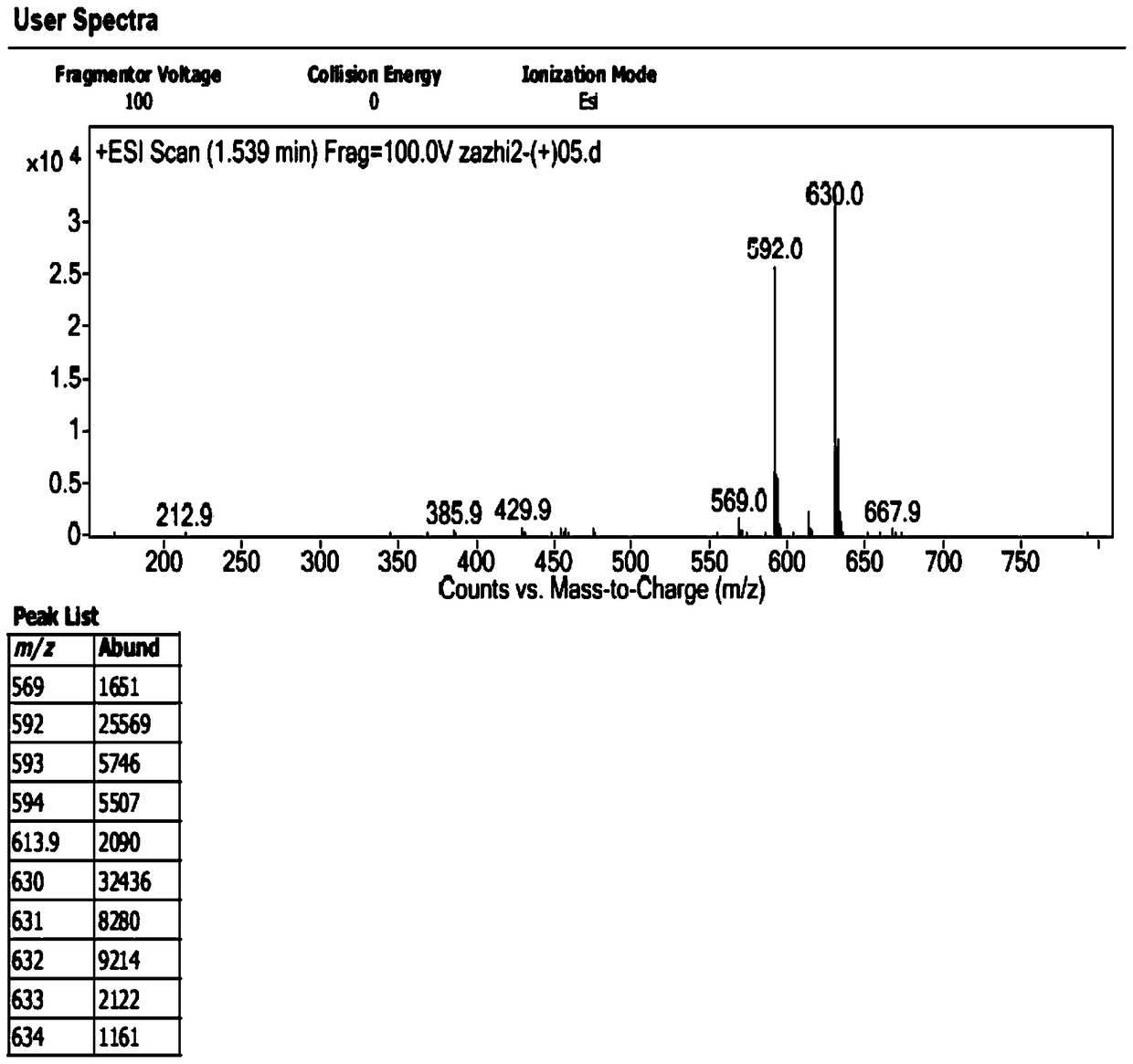
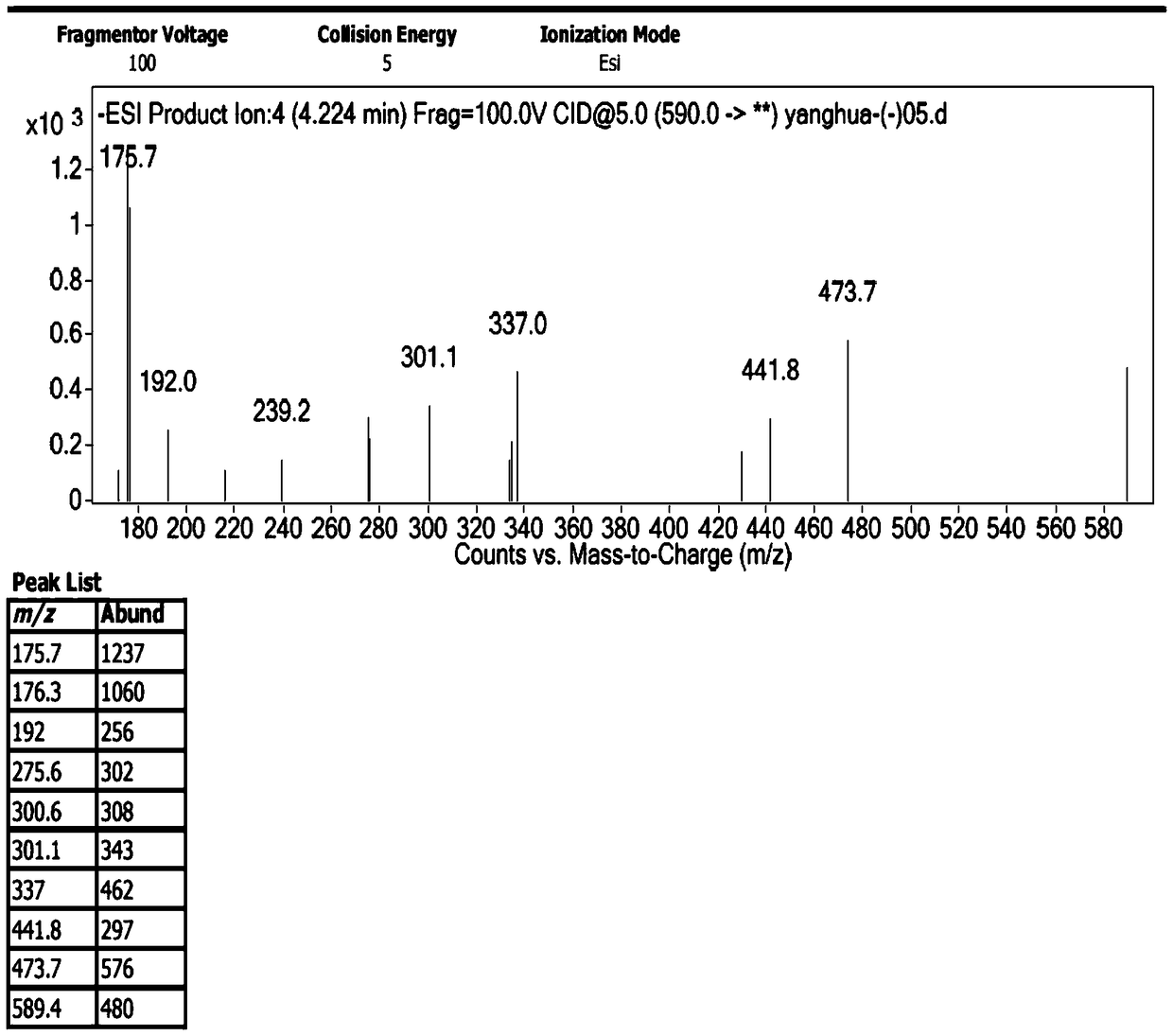
[0029] 1. Preparation of impurities
[0030] The chromatographic conditions are as follows: Agilent 1200 high performance liquid chromatography, Unitary C 18 100A (10mm×250mm, 10μm) semi-preparative column, with 0.1% trifluoroacetic acid aqueous solution-methanol-acetonitrile (80:10:10) as the mobile phase, take an appropriate amount of sample, dissolve it in water, and prepare 1.0g / ml impurity preparation For the test solution, the injection volume is 100 μl, the flow rate is 3.0ml / min, the detection wavelength is 254nm, and the column temperature is 30°C. Under the chromatographic conditions, the impurities peaked in about 7 minutes, and the cefotetan disodium peaked in about 20 minutes. After the impurities were detected by the ultraviolet detector, the fraction was collected after the column was started at the peak position, and the sample was enriched.
[0031] The collected fractions were concentrated under reduced pressure (15° C.) by a rotary evaporator for about 4 ho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com