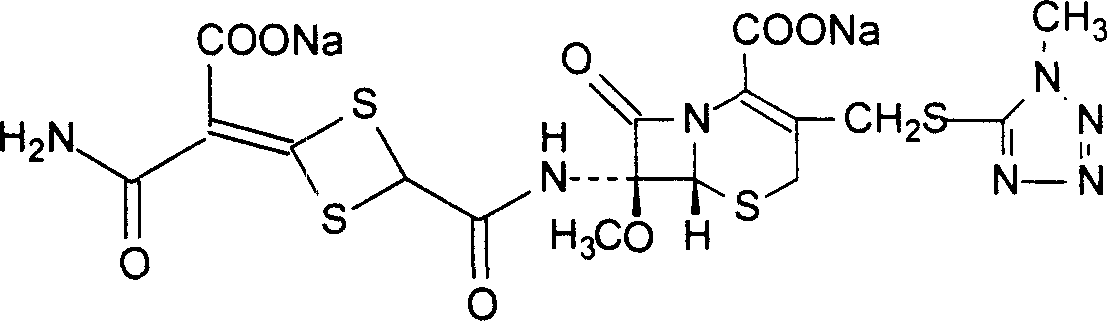
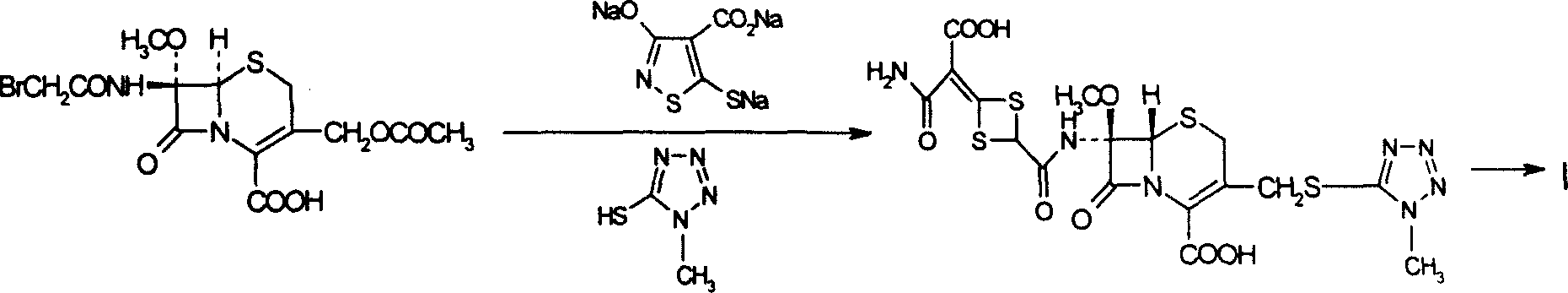
Method for preparing cefotetan bisodium
A technology of cefotetan disodium and cefene, which is applied in the field of preparation of beta-lactam antibiotic cefotetan disodium, can solve the problems of being unfavorable to large-scale industrialized production, high production cost, long route and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
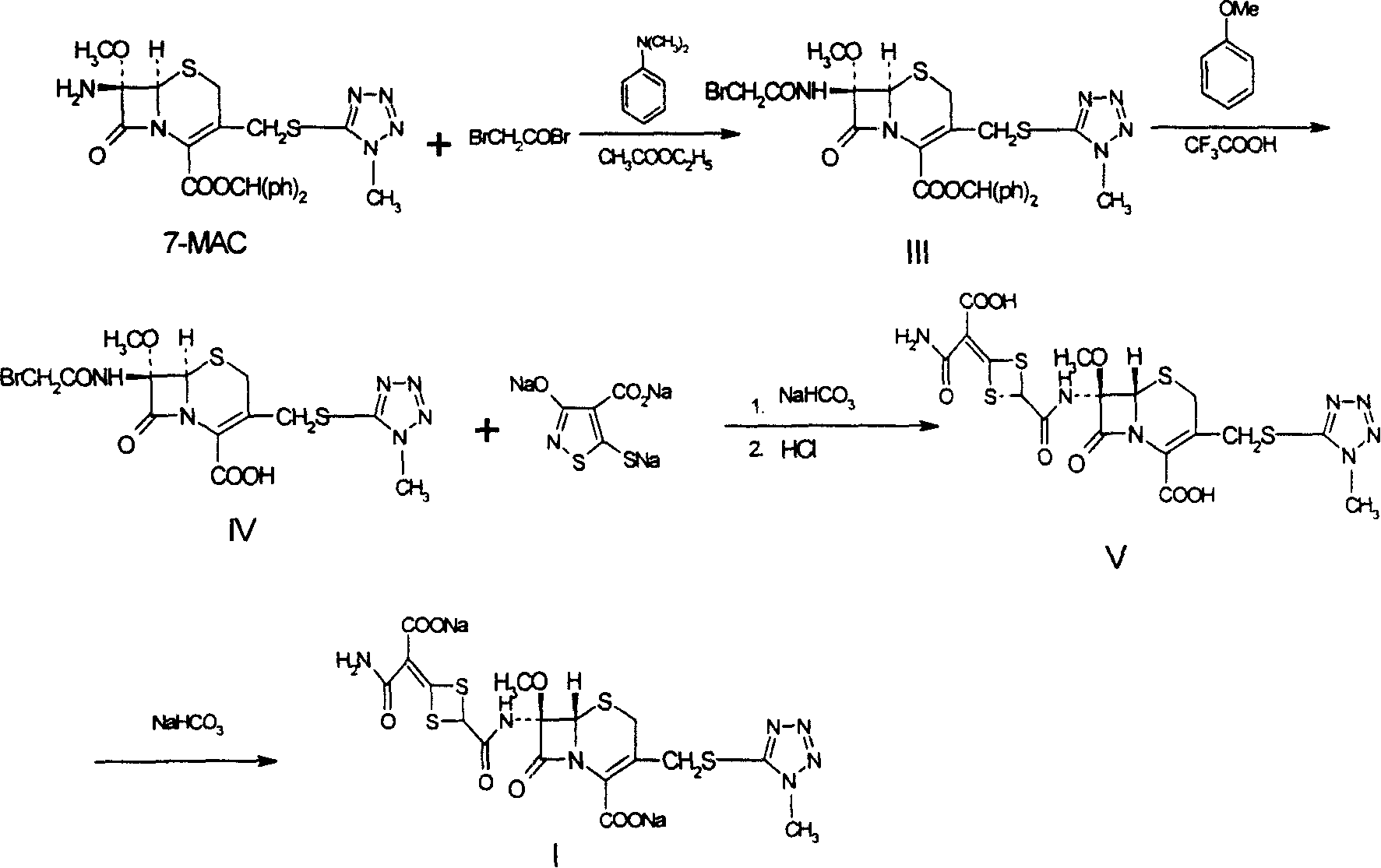
[0018] 1 Preparation of 7-β-bromoacetamide-7α-methoxyl group-3-(1-methyl-1H-5-tetrazolyl)thiomethyl-3-cephem-4-carboxylic acid benzhydryl ester ( III)
[0019]
[0020] ethyl acetate
140L
7-MAC
3.36kg
7mol
N,N-Dimethylaniline
840g
7mol
bromoacetyl bromide
1.4kg
7mol
0.1N hydrochloric acid
35L
Appropriate amount
[0021] Add 70L ethyl acetate, 7β-amino-7α-methoxy-3-(1-methyl-1H-5-tetrazolyl)thiomethyl-3-cephem-4-carboxylic acid di Benzyl ester (3.3kg, 7mol), stir to dissolve, cool in ice water to 0°C, add (840g, 7mol) N,N-dimethylaniline, add dropwise (1.4kg, 7mol) bromine under ice cooling Acetyl bromide, continue stirring at this temperature for 10 min, and add 35L of ice water and 70L of ethyl acetate to the reaction solution. The organic layer was washed three times with 12L of 0.1N hydrochloric acid solution, and dried over anhydrous mag...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com