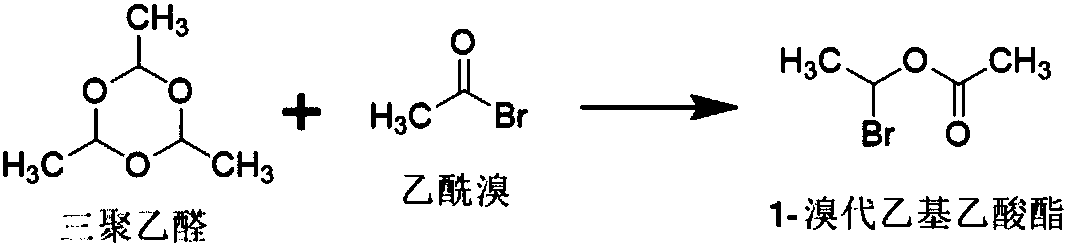
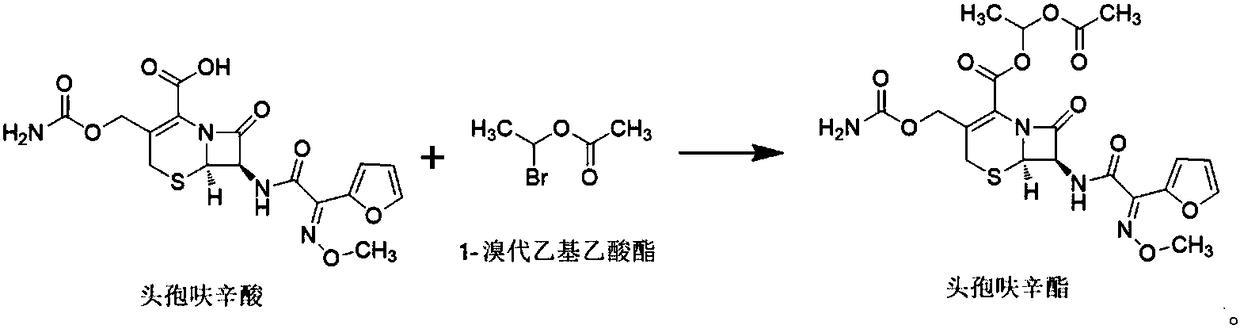
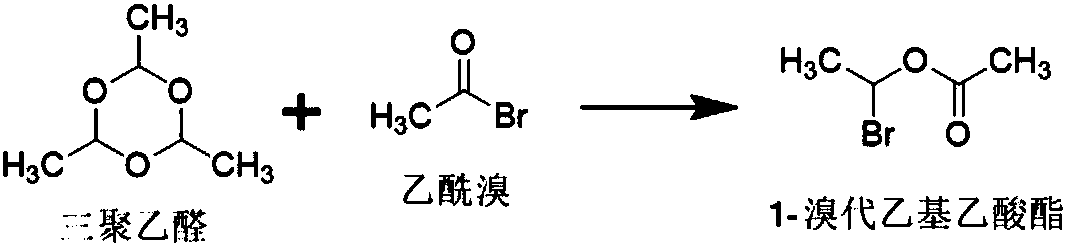
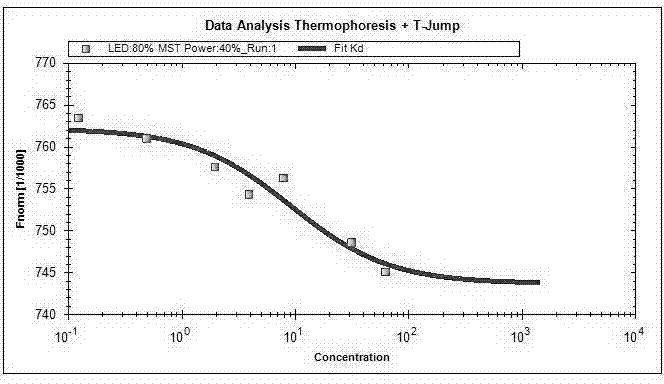
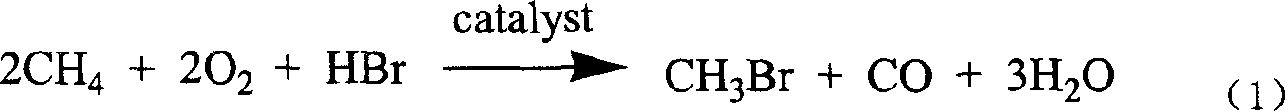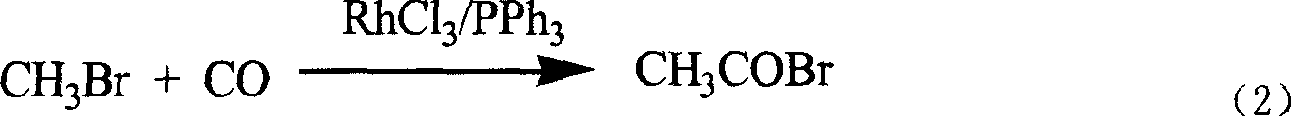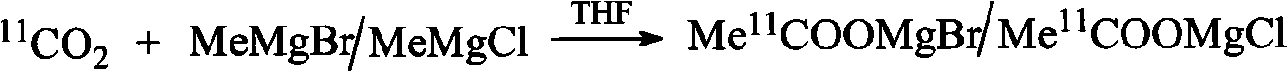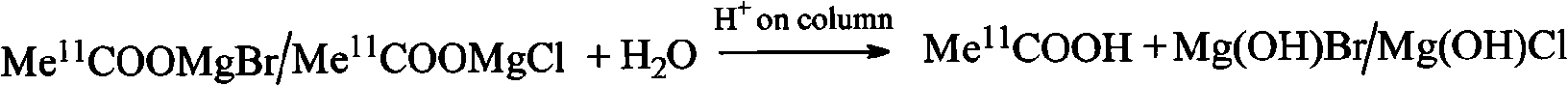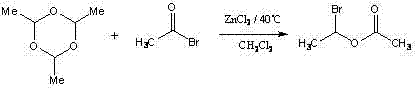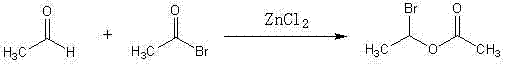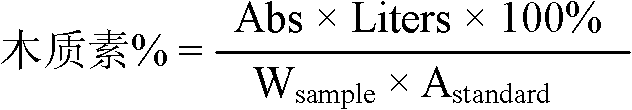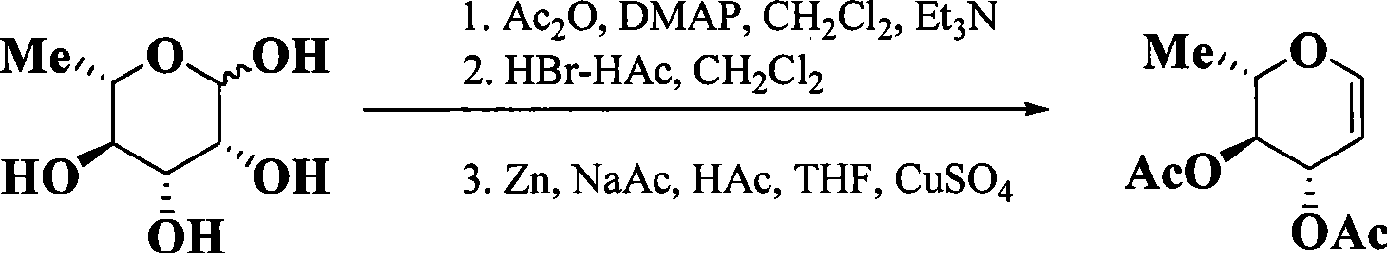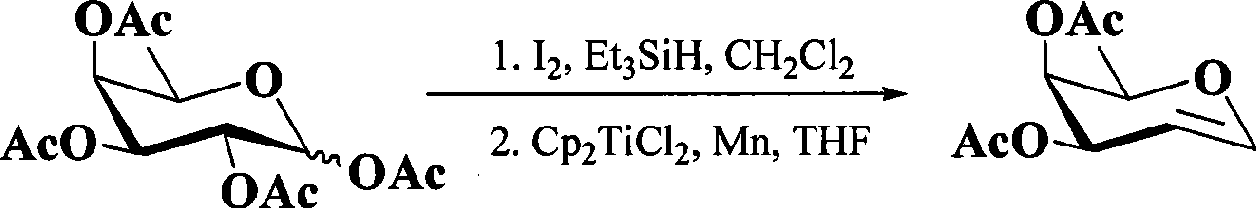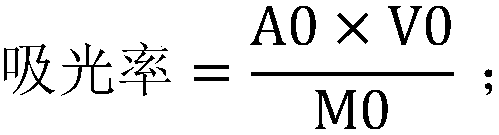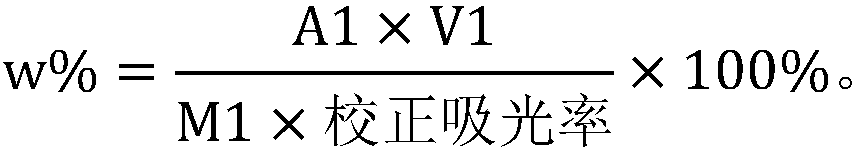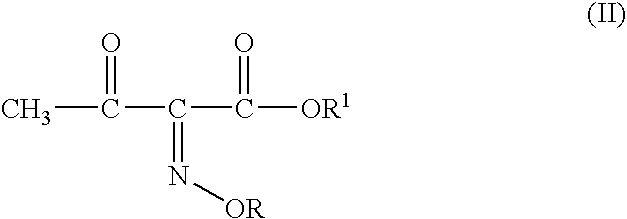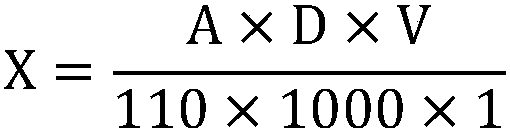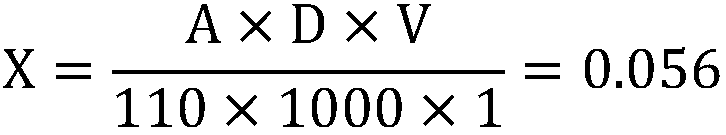Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
66 results about "Acetyl bromide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Acetyl bromide is an acyl bromide compound. As is expected, it may be prepared by reaction between phosphorus tribromide and acetic acid...
New technological process of synthesizing acetyl bromide, acetic acid, acetate from methane
ActiveCN1724503AEasily hydrolyzedReduces aggravated sedimentationPreparation from carboxylic acid halidesPreparation from carboxylic acid halideSolventOxygen
The invention discloses a novel process for preparing acetyl bromide, acetic acid and acetic ester from methane, which consists of reacting methane, oxygen and aqueous solution of HBr to obtain CH3Br and CO through catalytic reaction, then subjecting CH3Br and CO to carbonation reaction so as to prepare acetyl bromide in organic medium containing catalyst, then directly hydrolyzing the acetyl bromide and a certain amount of water into acetic acid, and reacting acetyl bromide and alcohols to obtain the acetic esters of the corresponding alcohols. The HBr can be regenerated as circular reaction medium and used again for the reaction for preparing CH3Br and CO from methane. The organic medium of the carbonylation reaction contains catalyst such as rhodium (Rh) compound, hydriodate and / or organo-phosphorus ligand, and a certain proportion of solvents.
Owner:MICROVAST POWER SYST CO LTD
Preparation method and use of main ingredient of fragrance releasing agent for tobacco with jasmine fragrance
InactiveCN101921297AReduce volatilityReduce fat solubilityTobacco treatmentSugar derivativesAdditive ingredientSilver carbonate
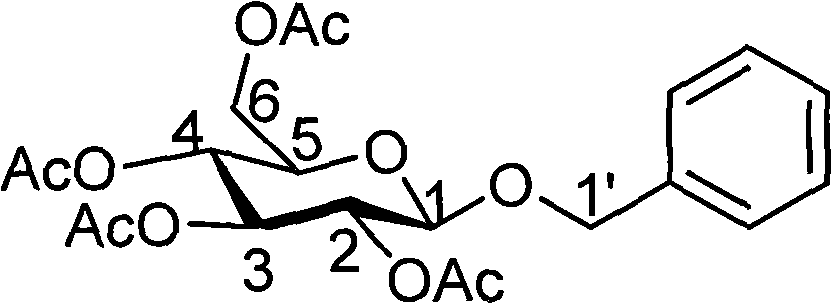
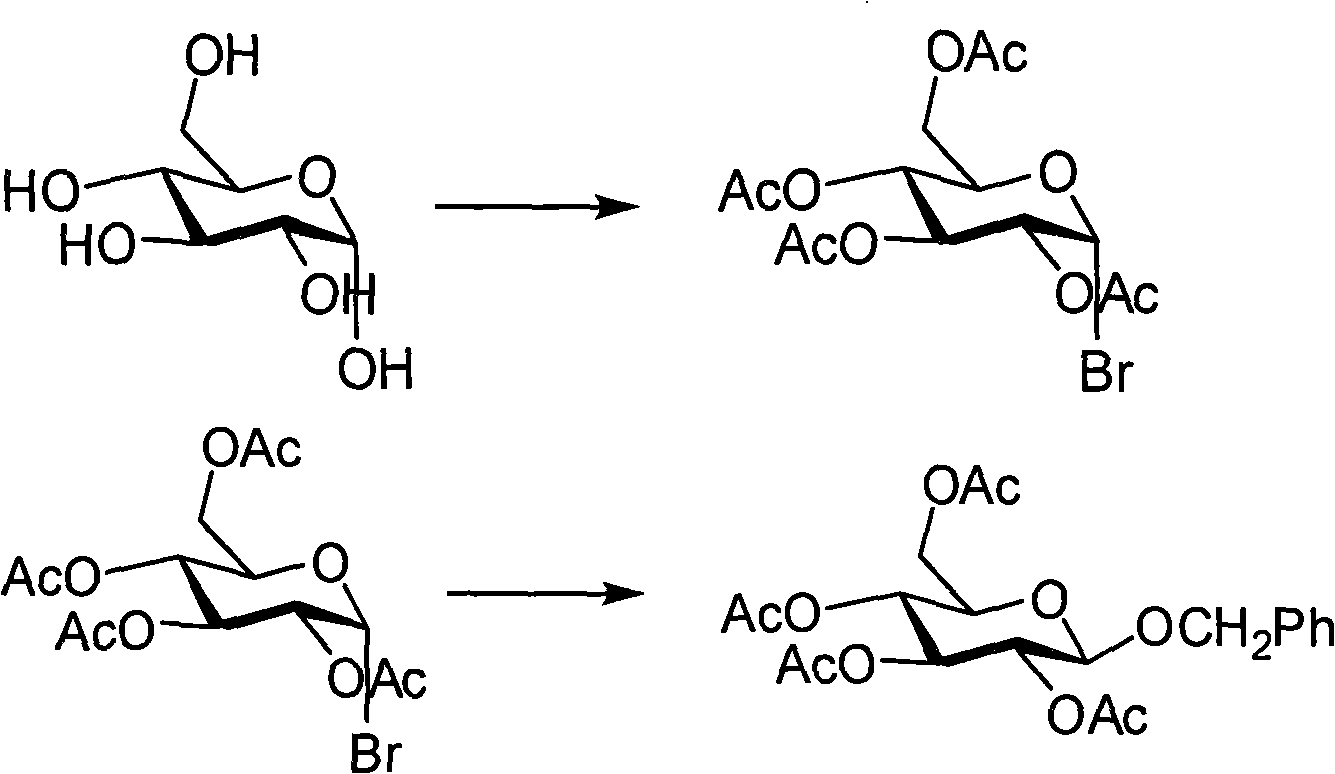
The invention discloses a preparation method and use of the main ingredient of a fragrance releasing agent for tobacco with a jasmine fragrance. The method comprises: preparing benzyl-2,3,4,6-tetra-o-acetyl-beta-d-glucopyranoside by a Cohen-Kohler reaction, acetylizing glucose in the presence of a perchloric acid catalyst, reacting the product of the acetylization with bromine hydride, removing solvent, recrystallizing in diethyl ether to obtain white crystals of alpha-acetobromoglucose; and reacting the alpha-acetobromoglucose with phemethylol and silver carbonate in a ratio of 1:2:1 in a dark place, removing the solvent, purifying by silica gel chromatography column and obtaining pure benzyl-2,3,4,6-tetra-o-acetyl-beta-d-glucopyranoside. In the invention, the benzyl-2,3,4,6-tetra-o-acetyl-beta-d-glucopyranoside is used as a main precursor with the jasmine fragrance. The fragrance releasing agent for tobacco with the jasmine fragrance, which is prepared by the invention, can release target fragrance effectively. The raw materials are simple and readily available. The cost is low, and the reaction equipment is simple.
Owner:CHINA TOBACCO FUJIAN IND
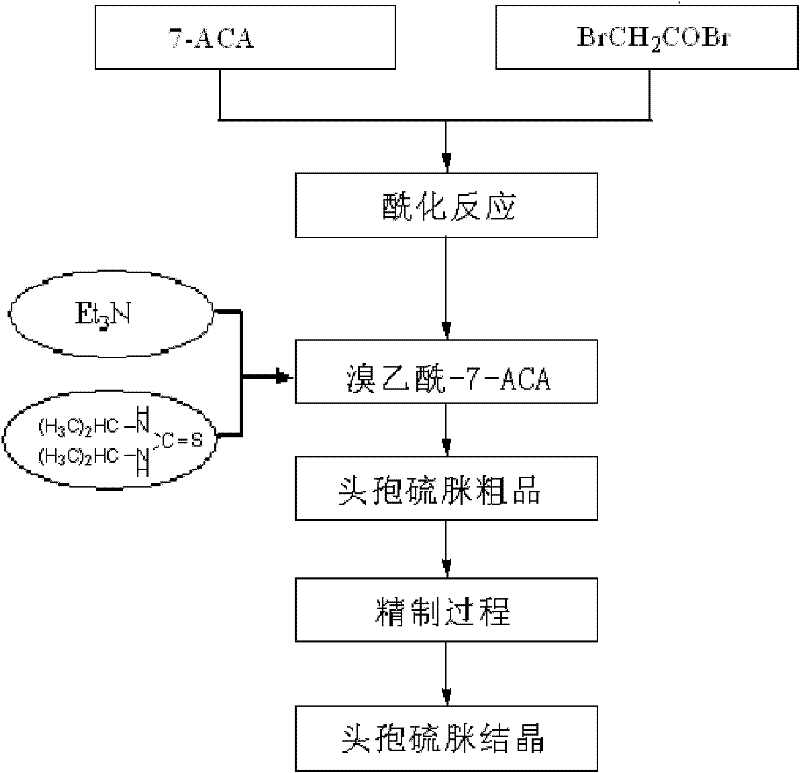
Preparation method of high-purity cefathiamidine
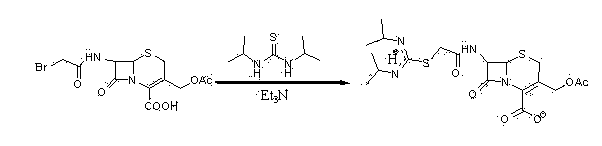
The invention relates to a preparation method of cefathiamidine, in particular to a preparation method of high-purity cefathiamidine with low content of impurity acetyl bromide-7-ACA (acetic acid). The preparation method comprises the following steps: adding acetyl bromide-7-ACA, a reaction solvent and less auxiliary solvent into a reactor, and adding alkali to completely dissolve the acetyl bromide-7-ACA; adding N,N'-di-isopropyl thiourea, reacting for 0.5 to 8.0 hours at the temperature between 0 and 40 DEG C; regulating the system temperature to be between 0 and 35 DEG C, dripping a crystallization solvent, then continuing to stir, filtering, cleaning, and drying to obtain the cefathiamidine. According to the preparation method in the invention, residues of the reactant acetyl bromide-7-ACA in cefathiamidine can be effectively reduced, the content of the acetyl bromide-7-ACA in the cefathiamidine is reduced to 0.2 percent or lower, and the content of the acetyl bromide-7-ACA can be reduced to lower than 0.1 percent or completely removed by virtue of further refining, so that the safety of the cefathiamidine is further improved.
Owner:GUANGZHOU BAIYUNSHAN PHARMA HLDG CO LTD BAIYUNSHAN PHARMA GENERAL FACTORY +1
C-acetate preparation method
InactiveCN101597228AReduce manufacturing costPreparation from carboxylic acid halideRadioactive preparation carriersBromineChloride
An C-acetate preparation method comprises the following steps: adopting methyl magnesium bromide or methyl magnesium chloride as precursor, reacting the precursor with CO2 in a Loop to prepare an intermediate C-acetyl bromine or magnesium chloride adduct; without adopting purification process, injecting N2 and removing tetrahydrofuran (THF), adding water in the mixture, injecting the mixture through integral small columns (C18 small column, SEP-PAK TSCX small column and SEP-PAK TIX small column) in turn for isolation and purification where the intermediate C-acetyl bromine or magnesium chloride hydrolyzes, filtrating by aseptic filter membrane, neutralizing by alkaline solution and obtaining C-AC parenteral solution. The preparation method of the invention has the advantages of the realization of column hydrolysis and purification, fully automatic synthesis, short synthesis time, high radiochemical yield, high radiochemical purity and low production cost.
Owner:THE FIRST AFFILIATED HOSPITAL OF SUN YAT SEN UNIV
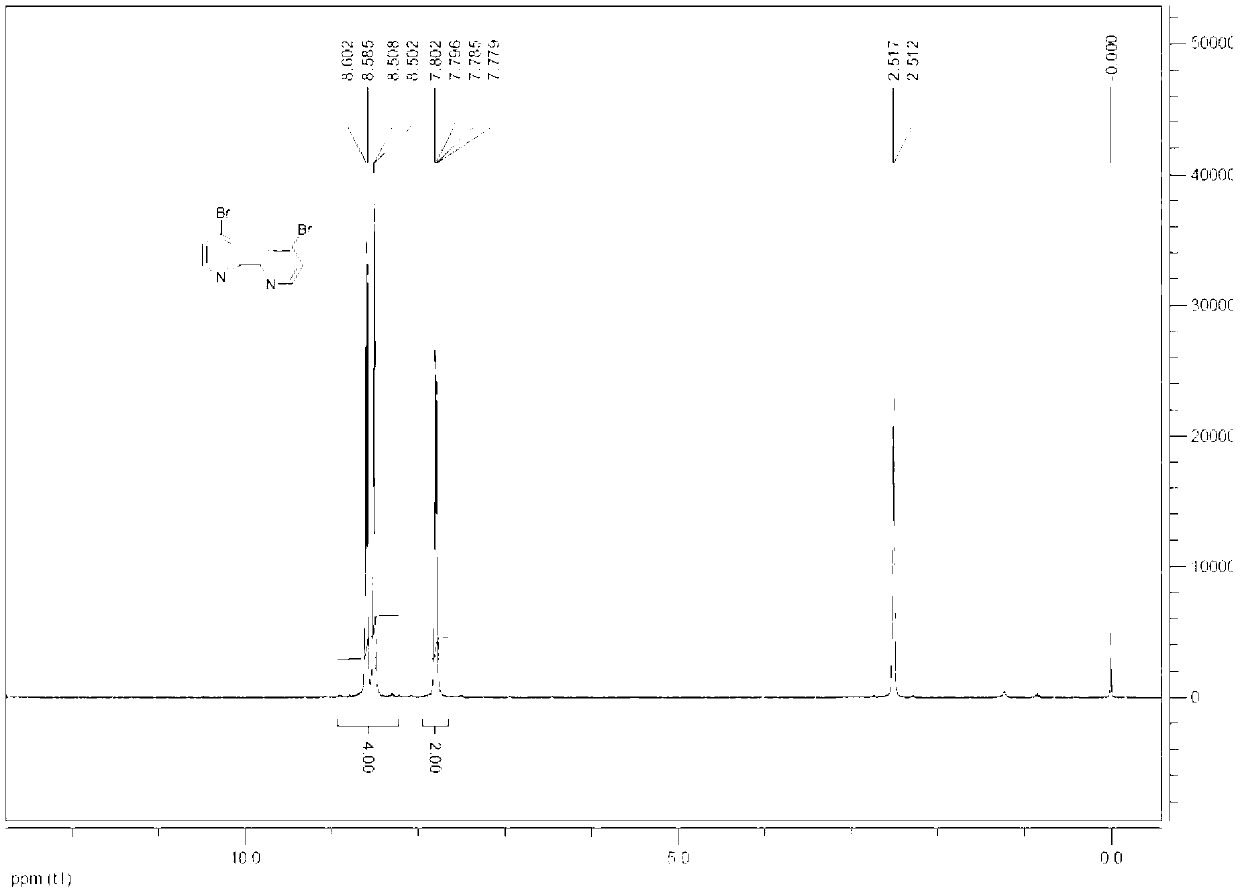
Synthesis method for 4, 4'-dibromo-2, 2'-dipyridyl
InactiveCN103130713AReduce dosageReduce adverse effectsOrganic chemistryPhosphorus tribromideSynthesis methods
The invention discloses a synthesis method for 4, 4'-dibromo-2, 2'-dipyridyl and belongs to the field of organic synthesis. The synthesis method includes the following steps: (A) oxidizing reaction: 2, 2'-dipyridyl reacts with 30% of hydrogen under the action of a metal catalyst and a phase transfer catalyst in the medium of water, and 2, 2'-dipyridyl nitrogen oxide is obtained; (B) nitration reaction: the 2, 2'-dipyridyl nitrogen oxide and fuming sulphuric acid are heated and react with fuming nitric acid in the medium of concentrated sulfuric acid, and 4, 4'-dinitro-2, 2'-dipyridyl nitrogen oxide is obtained; and (C) bromination reaction and deoxygenation reaction: the 4, 4'-dinitro-2, 2'-dipyridyl nitrogen oxide is added into acetic acid and heated till backflow, an acetyl bromide acetic acid solution is added, reaction is finished, a reducing agent is added, and the 4, 4'-dibromo-2, 2'-dipyridyl is obtained. According to the synthesis method for the 4, 4'-dibromo-2, 2'-dipyridyl, the solvent-free environment-friendly oxidizing reaction is adopted, the dosage of acetyl bromide is reduced, other reducing agents are used to replace phosphorus tribromide for the deoxygenation reaction, the bromination and reduction are changed into a one-pot method, harmful effects in the aspects of HSE (health, safety, environment) are reduced, and the synthesis method is environment-friendly and suitable for industrial production.
Owner:JIANGSU ZHONGDAN PHARMA RES +1
Substrate for detecting beta-D-glucuronidase and preparation method thereof as well as kit
InactiveCN108129530AStable in natureNot perishableSugar derivativesMicrobiological testing/measurementGlucuronateGlucuronidase
The invention discloses a substrate for detecting beta-D-glucuronidase. The substrate is specifically thymolphthalein-alpha-D-glucuronic acid. A preparation method comprises the following steps: preparing thymolphthalein-alpha-D-methyl glucuronate through reaction of thymolphthalein and acetyl bromide-alpha-D-methyl gluconate, removing acetyl to obtain the thymolphthalein-alpha-D-methyl gluconate,and then performing hydrolysis reaction, so as to obtain the thymolphthalein-alpha-D-glucuronic acid. As the substrate for detecting the beta-D-glucuronidase, the thymolphthalein-alpha-D-glucuronic acid disclosed by the invention has the advantages of stable property, difficulty in deterioration and sensitivity to development; furthermore, a synthesis process is simple, and the cost can be obviously reduced.
Owner:GUANGDONG UNITY BIOTECH +1
Preparation method of 2-deoxy-L-ribose
ActiveCN103694279AEasy to purifyHigh yieldSugar derivativesSugar derivatives preparationHydration reactionPolyolefin
The invention discloses a preparation method of 2-deoxy-L-ribose. The preparation method comprises the following steps: preparing beta-bromo-L-triacetyl arabinose by using L-arabinose as a starting material, acetyl bromide serving as a protecting group and a brominating reagent, adding the beta-bromine-L-tri-O-acetyl-arabinose into a reaction system containing zinc powder, sodium bisulfate, copper sulfate and water, reacting at normal temperature, and then diluting, filtering, washing, concentrating under reduced pressure and rectifying the reaction products to produce acetylated acetylated L-arabinal with the purity of larger than 99%; dissolving the acetylated acetylated L-arabinal into ethanol, adding potassium carbonate into the mixture to react, so as to generate L-arabinose olefin as an intermediate; performing hydration addition on the acetylated L-arabinal under composite catalyst action of a sulphoalkyl pyrazine sulphate-long-chain polyolefin sulfonic acid resin, so as to produce the 2-deoxy-L-ribose. The used raw materials are conventional reagents, while the reaction conditions are milder, the operation method and the post-treatment processes are simple, and the product yield and purity are relatively desirable.
Owner:JIANGXI SUKEER NEW MATERIAL
Method for synthesizing (R,S)1-bromoethyl acetate
ActiveCN102417451AReduce generationEmission reductionOrganic compound preparationCarboxylic acid esters preparationBromineSolvent
The invention relates to a method for synthesizing (R,S)1-bromoethyl acetate. The method comprises the following steps of: dissolving acetaldehyde and acetyl bromide into a solvent, and reacting in the presence of a catalyst with a naphthalene ring structure to obtain the (R,S)1-bromoethyl acetate. By the method, the content and yield of the (R,S)1-bromoethyl acetate are remarkably improved; the process is simple and can be operated stably; three wastes (waste gas, waster water and industrial residues) are reduced; the cost is reduced; the content and yield of cefuroxime axetil are remarkably improved when the method is applied to the production; and the method is suitable for industrialized production.
Owner:ZHEJIANG GUOBANG PHARMA
Process for preparing high-purity cefathiamidine
The present invention relates to a process for preparing cefathiamidine, in particular a process for preparing high-purity cefathiamidine. The preparation method comprises the following steps of: adding water or an aqueous inorganic salt solution as an auxiliary solvent in or after a reaction on acetyl bromide-7ACA and N,N'-diisopropylthiourea; after the reaction, cooling the mixture, and dropwise adding a crystallization solvent into the mixture, and performing suction filtration to obtain a crude product of cefathiamidine; and purifying the crude product again,and drying the crude product in vacuum to obtain the high-purity cefathiamidine. According to the preparation method provided by the present invention, residues of impurities in the cefathiamidine can be effectively reduced, the content of acetyl bromide-7ACA and the macromolecular compound impurities can be reduced to 0.1% or less or can be completely eliminated, and other residues of impurities in the cefathiamidine can be reduced to be less than 0.2%.
Owner:山西振东泰盛制药有限公司
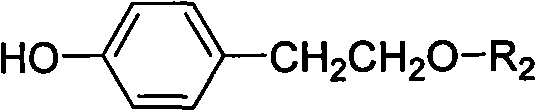
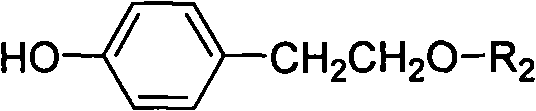
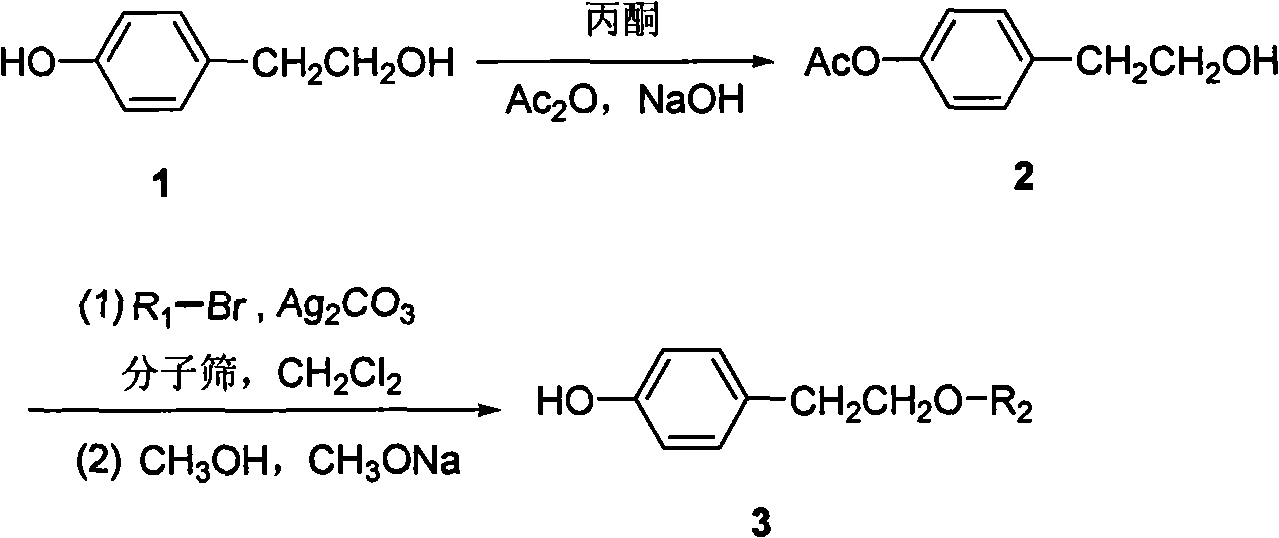
Method for preparing tyrosol glucoside in quantities
InactiveCN101654468AShort synthetic stepsMild reaction conditionsSugar derivativesSugar derivatives preparationTyrosolSugar substitute
The invention discloses a method for preparing tyrosol glucoside in quantities; in the method, tyrosol is taken as raw material, and tyrosol phenolic hydroxyl acetylation protection is carried out selectively to be condensed with acetyl bromide sugar substitute followed by de-protection. The invention features available raw material, less reaction steps, high yield, simple preparation technique, stable technical parameters, capability of preparing kilogram level high purity (more than 98%) tyrosol glucoside once, and application in preparing various tyrosol glucoside.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Method for detecting and analyzing lignin content of pear pulp
ActiveCN102608054AEasy to get out of waterReduce connectivityPreparing sample for investigationColor/spectral properties measurementsPEARBromine
The invention discloses a method for detecting and analyzing lignin content of pear pulp. The method comprises the following steps of: freezing the pear pulp to be detected, homogenizing, crushing, sieving, cleaning to remove soluble impurities, and digesting and hydrolyzing pear pulp cells by use of acidification acetyl bromide to dissolve lignin; then adding sodium hydroxide and hydroxylamine hydrochloride into the digested and hydrolyzed solution to terminate the reaction, and centrifuging the solution to eliminate the influence of impurities and precipitates on absorbance measurement; and finally measuring the absorbance value at 280 nanometers by using an ultraviolet spectrophotometer, and substituting the absorbance value into a formula to calculate the lignin content. An analysis method which has the advantages of accurate analysis result, high analysis speed, simplicity in operation, high sensitivity and good reproducibility is provided for the relatively low lignin content of the pear pulp.
Owner:ANHUI AGRICULTURAL UNIVERSITY
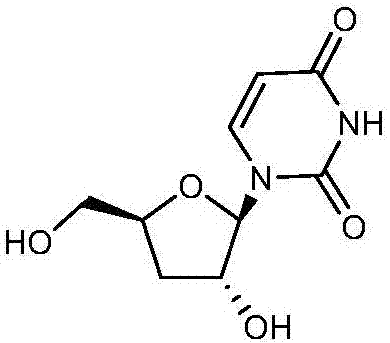
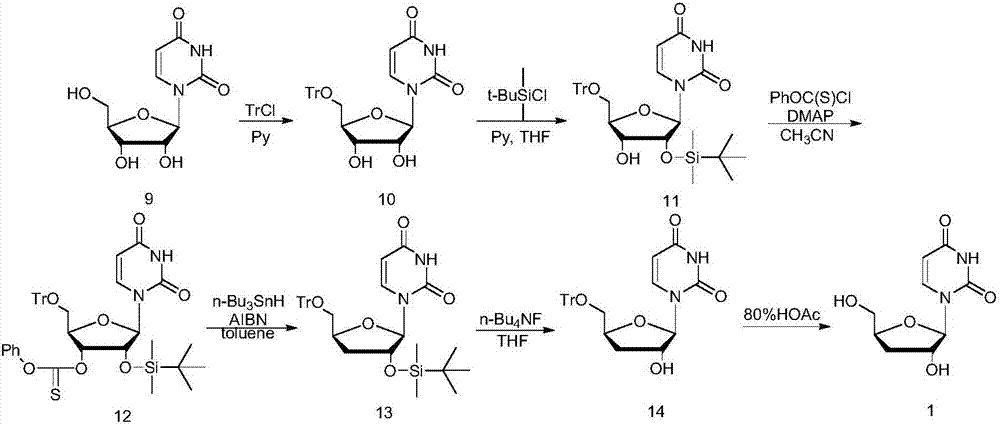
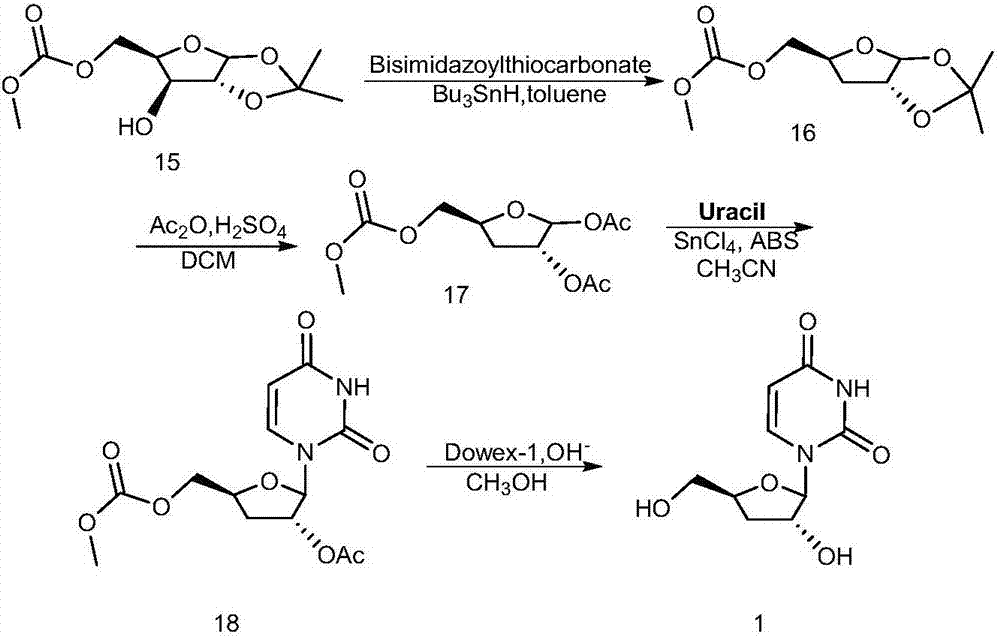
Preparation method of 3'-deoxyuridine
ActiveCN107033205AReduce usageAvoid generatingSugar derivativesSugar derivatives preparationAcetic anhydrideHigh pressure water
The invention relates to the field of pharmaceutical synthesis and particularly relates to a preparation method of 3'-deoxyuridine. The method comprises the steps: by adopting a compound 3 as a raw material, firstly protecting amino through acetic anhydride to obtain a compound 4, obtaining a compound 5 under the action of acetyl bromide, reducing through a hypophosphite system to obtain a compound 6; removing deacetylated amino under the action of high-pressure water vapor and an organic solvent to obtain a compound 8 or removing N-acetyl to obtain a compound 7; and finally removing all acetyl to obtain a mixture of 3'-deoxyuridine and 3'-deoxycytidine; separating and purifying to obtain 3'-deoxyuridine crystal and 3'-deoxycytidine crystal separately, or directly removing all acetyl through the compound 6 to obtain the 3'-deoxycytidine. Available natural products are taken as initial raw materials, so that the method is simple in operation and convenient to purify, and industrial large-scale production is extremely easy to implement.
Owner:SHANGHAI ZHAOWEI TECH DEV +1
New synthesis method of pseudouridine
PendingCN114702481AReduce usageFew reaction stepsOrganic chemistry methodsFermentationAcetic anhydrideReaction step
The invention discloses a novel synthesis method of pseudouridine, which comprises the following steps: 1) taking D-ribose as a reaction initiator, and dropwise adding acetyl bromide into an organic solvent to obtain an intermediate I; 2) using uracil as a reaction initiator, eliminating reactive hydrogen in an organic solvent by using strong Lewis base, and adding (Boc) 2O in batches to obtain a corresponding intermediate II; and (3) carrying out condensation reaction on the compound I and the compound II in an organic solvent under the condition of strong Lewis base to obtain an intermediate III, removing a protecting group by using trifluoroacetic acid to obtain an intermediate IV, adding acetic anhydride, carrying out chiral resolution by using lipase to obtain a compound V, and finally removing a protecting group under an alkaline condition to obtain a final product VI. The product is a pseudouridine product. According to the method, mild and safe chemical reagents and enzyme chiral resolution are adopted, the reaction steps are shortened, the reaction difficulty is reduced, and therefore the technical bottleneck of green synthesis is achieved.
Owner:南京艾斯特医药科技有限公司
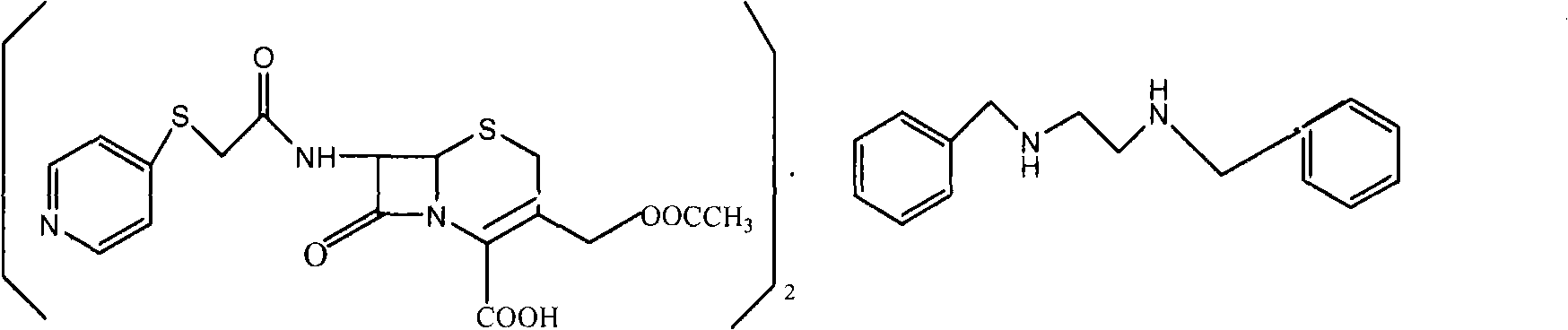
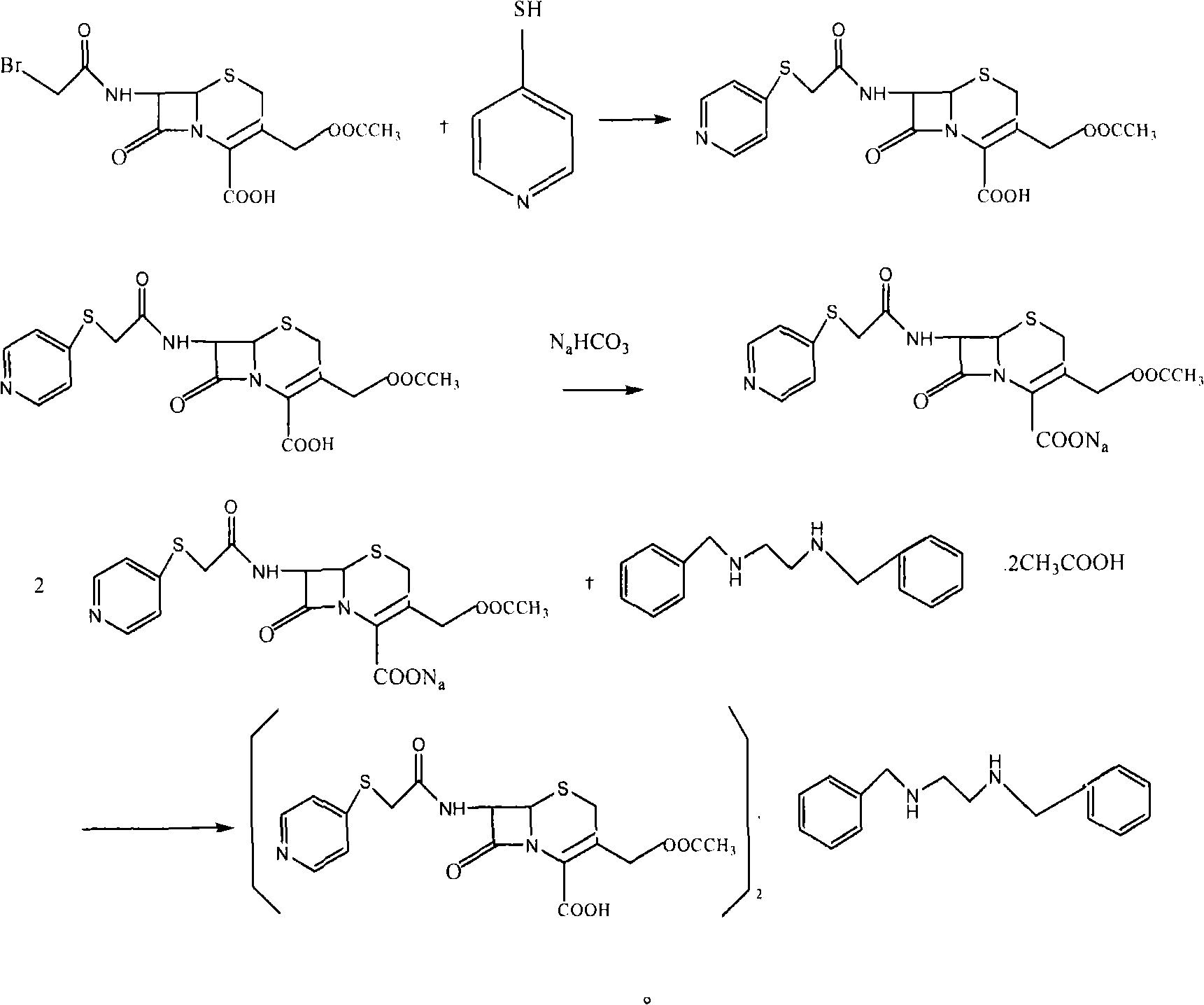
Method for preparing cephapirin benzathine
The invention discloses a new method for preparing cephapirin benzathine by a one-pot method, relating to the field of chemical drug synthesis. The method comprises the following steps of: taking acetyl bromide 7-ACA as raw materials; in a solvent, in the presence of organic alkali, reacting the acetyl bromide 7-ACA with 4-pyridinethiols to obtain solid cefapirin acid; and then adding the organic alkali in the reaction liquid, dissolving the cefapirin acid as salt, and simultaneously and unnecessarily adding a reductant which is sodium thiosulfate, sodium hydrogensulfite or vitamin C; and then adding a reagent A in the reaction liquid, reacting the cefapirin acid salt with the reagent A at 0-50 DEG C for 0.1-20 hours to obtain the cephapirin benzathine, wherein the reagent A is dibezylethylenediamine diacetate acetate or dibezylethylenediamine diacetate. The method solves the problems that a product is easily decomposed, color is easily deepened in the process of preparing cefapirin sodium salt, and the like. The invention is simplified in steps, easy to operate and suitable for industrialized production, and the quality of the product accord with the quality standard of USP29.
Owner:GUANGZHOU BAIYUNSHAN PHARM CO LTD
Crystalline cefuroxime axetil preparation method
InactiveCN108586493AEasy to manufactureEasy to purifyOrganic chemistryOrganic solventCefuroxime axetil
The invention relates to a crystalline cefuroxime axetil preparation method, which comprises: 1) carrying out a reaction on paraldehyde and acetyl bromide at a temperature of -10-10 DEG C under the action of a catalyst to obtain 1-bromoethyl acetate; 2) adding cefuroxime acid and a catalyst to the mixed solution of an organic solvent and water, adjusting the temperature to -20-5 DEG C, adding the1-bromoethyl acetate prepared in the step 1) to the mixed solution in a dropwise manner to obtain the crystalline cefuroxime axetil. According to the present invention, the method improves the traditional process, simplifies the process, improves the product quality, does not use the extraction solvent dichloromethane, greatly reduce the production cost, and improves the reaction efficiency and the purity of the final product.
Owner:BENGBU BBCA MEDICINE SCI DEV
Method for ultrasonically synthesizing glycal
InactiveCN101935313AEliminate separation and purification workLow costSugar derivativesSugar derivatives preparationAcetic anhydrideOrganic synthesis
The invention relates to pharmaceutical chemistry and organic synthesis technology, in particular to a method for ultrasonically synthesizing glycal, such as acetylated glucal or acetylated maltose, and solves the problems of high cost, complex intermediate product processing, need of separation and purification, low yield and the like in the glycal preparation method. The method comprises the following steps of: preparing D-acetylated glucose or D-acetylated maltose from D-glucose and / or D-maltose and acetic anhydride under the catalytic action of perchloric acid or benzene sulfonic acid; directly adding the produced D-acetylated glucose or D-acetylated maltose into acetyl bromide and methanol to obtain 1-bromoacetylglucose and / or 1-bromoacetylmalcose; adding the obtained bromoacetylglucose or bromoacetylmalcose into aqueous solution of beta-cyclodextrin; adding zinc powder into the solution; and performing ultrasonic oscillation reaction to obtain the D-acetylated glucal and / or D-acetylated maltose. The method has the advantages of reducing the cost, along with complete reaction and yield of over 90 percent.
Owner:SHANXI AGRI UNIV
Preparation method of ulipristal acetate bulk drug impurities
The invention belongs to the technical field of steroid hormone drug preparation, and particularly relates to an ulipristal acetate bulk drug impurity preparation method, which comprises: dissolving ulipristal acetate in a solvent, carrying out a reaction under acetic anhydride and acetyl bromide conditions, and treating to obtain the ulipristal acetate bulk drug impurity, wherein the chemical name of the ulipristal acetate bulk drug impurity is 17 alpha-acetoxy-3-acetoxy-11beta-[4-(N,N-dimethylamino)phenyl]estra-1,3,5(10)-trien-20-one, the reaction process is defined in the specification, thesolvent is dichloromethane, and the mole number of acetic anhydride is 0.5-3 times that of ulipristal acetate. The product provided by the invention is high in purity and high in yield, and is used as a reference substance in an ulipristal acetate production process for controlling the quality of ulipristal acetate.
Owner:HUNAN NORCHEM PHARMACEUTICAL CO LTD
Preparation method of 1-bromoethyl acetate
InactiveCN103012143ALow Feed QuantityLow costPreparation from carboxylic acid halidesParacetaldehydeCombinatorial chemistry
The invention discloses a preparation method of 1-bromoethyl acetate, which comprises the following steps: under the catalysis of aluminum oxide, reacting acetyl bromide with paracetaldehyde; and then, extracting with dichloromethane / water to obtain the high-purity 1-bromoethyl acetate. According to the method, the paracetaldehyde is used instead of acetaldehyde in the traditional process, and the aluminum oxide is used for catalysis preferably, so that the method is environment-friendly, is easy to realize quantification and ensures high yield and purity of the product; and meanwhile, the process conditions of the method are mild and easy to control, thereby being suitable for industrial production.
Owner:GUANGDONG LIGUO PHARMACY
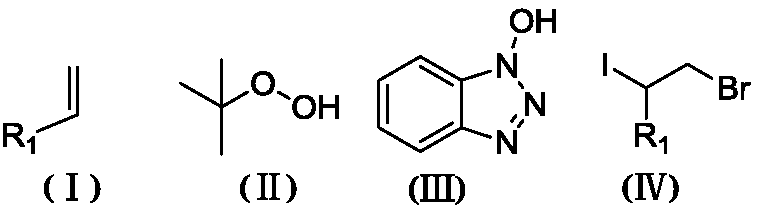
Method for synthesizing 2-bromo-1-iododihalide by one-pot process
InactiveCN108503500AMild reaction conditionsGood substrate adaptabilityCarboxylic acid nitrile preparationOrganic compound preparationEnvironmental resistanceOrganic solvent
The invention discloses a method for synthesizing 2-bromo-1-iododihalide represented by the formula (IV). The method comprises the following steps: mixing an olefin compound represented by formula (I)with an iodine source, tert-butyl hydroperoxide represented by formula (II) and N-hydroxybenzotriazole represented by formula (III) in an organic solvent, and completely reacting the obtained solution at a temperature from room temperature to 50 DEG C to obtain a reaction solution A; and adding acetyl bromide to the reaction solution A, completely reacting the obtained solution at room temperature to obtain a reaction solution B, and post-treating the reaction solution B to obtain the 2-bromo-1-iododihalide. The method has the advantages of safety, environmental protection, no generation of waste gas, facilitation of application in pharmaceutical synthesis, mild reaction conditions, good substrate adaptability, and realization of corresponding 2-bromo-1-iododihalides from various substituent groups.
Owner:ZHEJIANG UNIV OF TECH
Preparation of 3,4-di-O-acetyl-L-rhamnal
The invention belongs to the technical field of organic chemistry, and particularly relates to a method for preparing 3,4-di-O-acetyl-L-rhamnal. The method comprises that: firstly, under the catalytic action of p-toluenesulfonic acid(perchloric acid, amido-sulfonic acid, zinc chloride and sulphuric acid), L-rhamnal reacts with acetic acid and acetic anhydride to obtain 1,2,3,4-tetra-O-acetyl-L-rhamnose; the 1,2,3,4-tetra-O-acetyl-L-rhamnose is added with acetyl bromide and methanol for reaction to generate 1-bromine-2,3,4-tri-O-acetyl-L-rhamnose; and the 1-bromine-2,3,4-tri-O-acetyl-L-rhamnose is added with ethyl acetate(acetone), an aqueous solution of disodium hydrogen phosphate(a phosphate buffer and a solution of potassium phosphate dibasic) and the zinc powder for reaction to generate the 3,4-di-O-acetyl-L-rhamnal. The method has the characteristics of easy operation, short process flow, high yield, short reaction time, low cost, little 'three wastes', little environmental pollution, easy industrialization and the like.
Owner:CHENGDU INST OF BIOLOGY CHINESE ACAD OF S
Acetyl bromide synthesis production process
InactiveCN104086403AEasy to useEasy to prepareOrganic compound preparationCarboxylic compound preparationAcetic anhydrideBromine
The invention relates to an acetyl bromide synthesis production process, and relates to the technical field of chemical engineering. According to the invention, acetyl bromide is obtained through a bromination reaction of acetic anhydride and liquid bromine. The process provided by the invention has the following advantages: preparation is convenient and simple; the process is environment-friendly and pollution-free; raw materials are easy to obtain; equipment investment is low; product purity is high; operation is convenient; the prepared acetyl bromide has good application effect; and the prepared acetyl bromide is safe and reliable.
Owner:蚌埠团结日用化学有限公司
Chemical synthesis process for preparing gastrodin and similar phenolic glucoside thereof
InactiveCN105646610AIncrease conversion rate per passHigh yieldSugar derivativesSugar derivatives preparationChemical synthesisAcetic anhydride
The present invention discloses a chemical synthesis process for preparing gastrodin and similar phenolic glucoside represented by a formula (I) thereof. According to the present invention, industrial easily-available raw materials such as acetic anhydride and acetyl bromide or PHOSPHORUS TRIBROMIde or phosphorus pentabromide are used to complete the hydroxy protection of glycosyl and the bromination reaction of hemiacetal hydroxy, such that the problems that the use of red phosphorus and bromine during the gastrodin bulk drug production process causes physical and psychological harm on production workers and environment pollution and damage are solved; and the process mainly comprises two steps of key reactions, wherein the first step reaction is synthesis of bromoacetyl glycosyl compound, and the second step reaction is condensation of the bromoacetyl glycosyl compound and a phenolic compound to generate the acetyl-protected phenolic glucoside compound.
Owner:赵建英
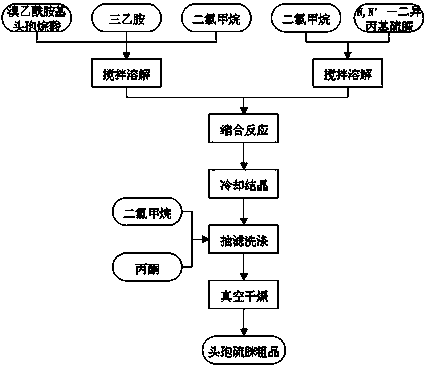
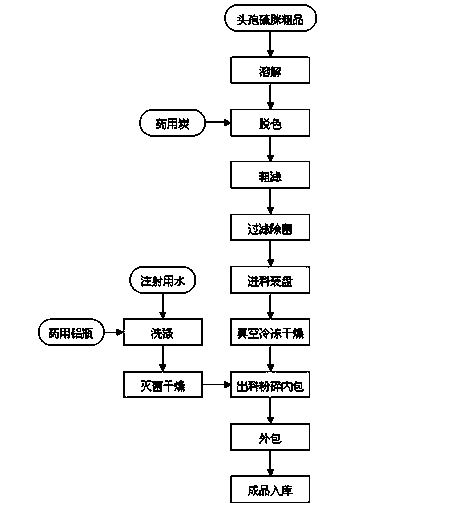
Preparation technology for cefathiamidine
The invention discloses a preparation technology for cefathiamidine. The preparation technology specifically comprises the steps that dichloromethane and N,N'-Diisopropyl thiourea are evenly stirred, dichloromethane, acetyl bromide aminocephalosporanic acid and triethylamine are stirred and dissolved, the materials are sucked into the same reaction kettle after dissolution, a condensation reaction is carried out, then crude cefathiamidine is obtained after cooling crystallization, suction filtration washing and vacuum drying, and the crude cefathiamidine is refined through lyophilization to obtain the finished cefathiamidine. The technology is stable, the high-quality and high-purity cefathiamidine can be obtained, and the yield can reach 80.0% to 88.0%.
Owner:JIANGSU HI STONE PHARMA
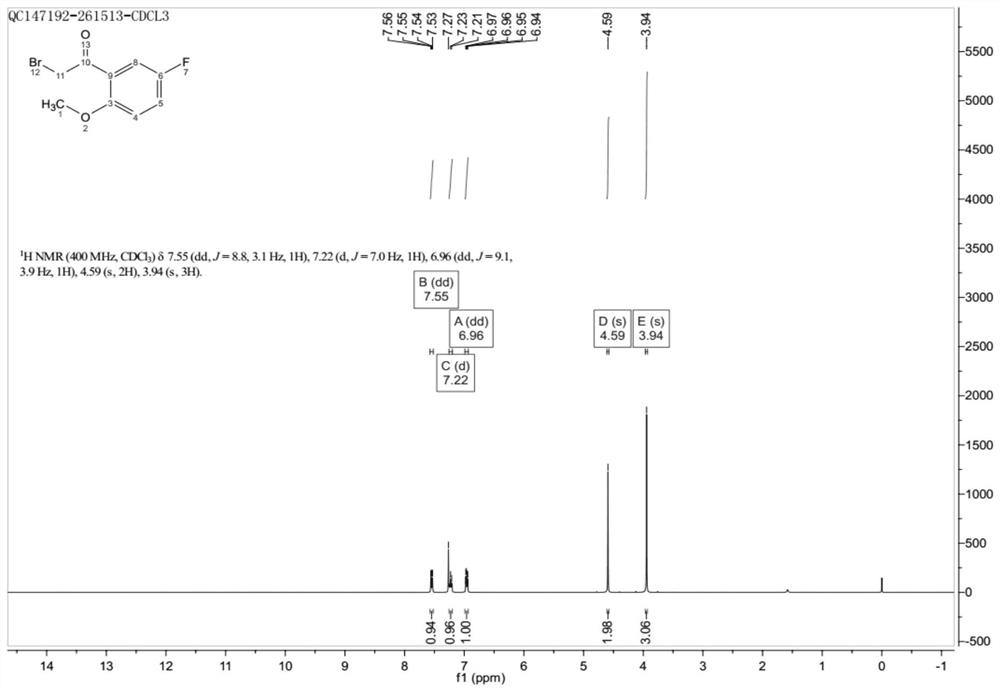
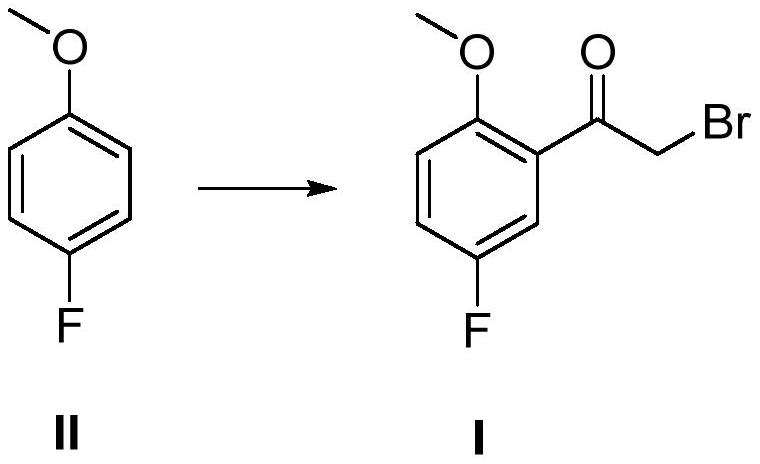
Preparation method of 2-methoxy-5-fluorobromoacetophenone
ActiveCN113354521ALow priceReduce pollutionCarbonyl compound preparation by condensationBulk chemical productionBiochemical engineeringCombinatorial chemistry
The invention discloses a preparation method of 2-methoxy-5-fluorobromoacetophenone. The method comprises the following steps: taking p-fluoroanisole as an initial raw material, and reacting with bromoacetyl bromide under the condition of Lewis acid to generate a compound I, namely 2-methoxy-5-fluorobromoacetophenone. In the one-step synthesis route process taking the p-fluoroanisole as the initial raw material, a large amount of reagents with high toxicity and high pollution are avoided, and meanwhile, the raw materials are low in price, so that the whole synthesis process is low in pollution and easy to treat.
Owner:无锡双启科技有限公司
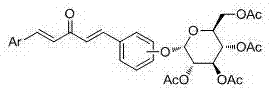
Glucoside-1,4-pentadiene-3-ketone compounds containing acetoxyl groups as well as preparation method and use of glucoside-1,4-pentadiene-3-ketone compounds
ActiveCN104497067AGood inhibitory effectNovel structureBiocideSugar derivativesTobacco mosaic virusCucumber mosaic virus
The invention discloses a preparation method of compounds for preventing and controlling plant virus disease: glucoside-1,4-pentadiene-3-ketone compounds containing acetoxyl groups and bioactivity, namely the compounds shown in the general formula (b) and a preparation method thereof. According to the preparation method, the glucoside-1, 4-pentadiene-3-ketone compounds containing the acetoxyl groups are synthesized by taking acetone, 2 or 4-substituted hydroxybenzaldehyde, substituted arylamine and acetyl bromoglucose as raw materials, ethanol and dichloromethane as solvents, dichloromethane as a catalyst and tetrabutylammonium bromide as a phase transfer catalyst. The compounds (f6), (f8), (f10), (f26) and (f28) have relatively good inhibitory effects on tobacco mosaic virus, cucumber mosaic virus and the like. The formula is shown in the description.
Owner:GUIZHOU UNIV
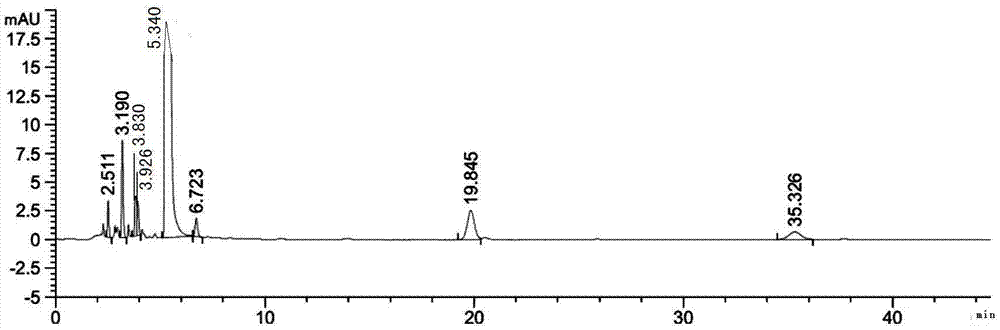
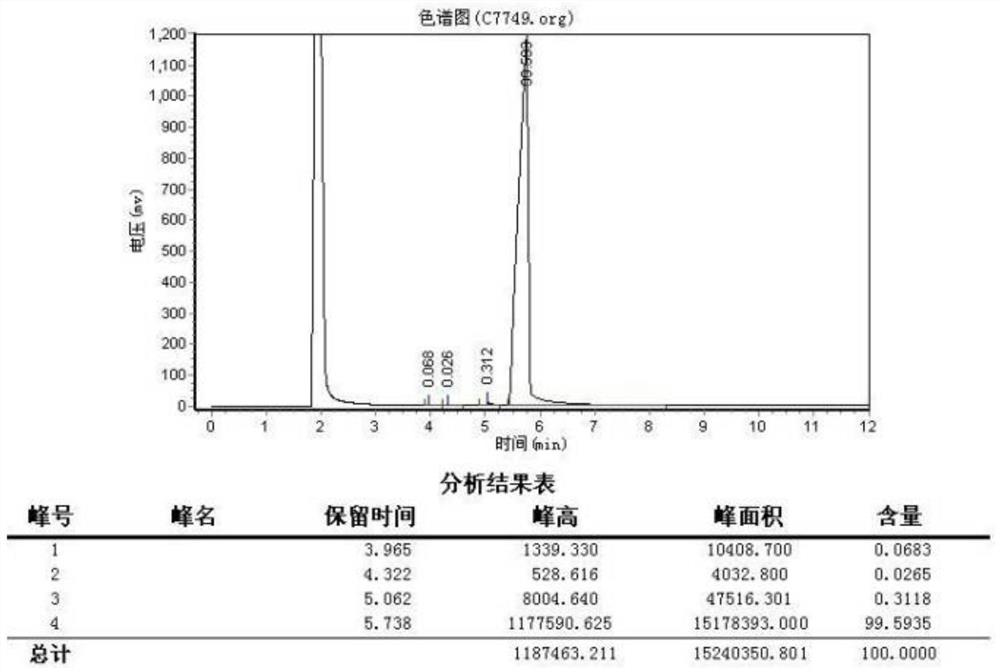
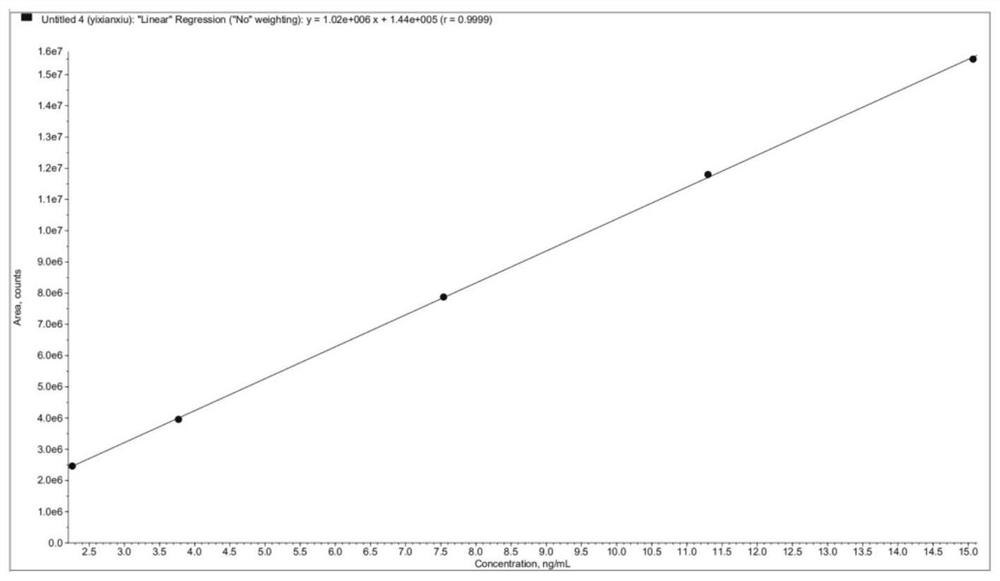
Method for analyzing content of acetyl bromide in cefuroxime axetil
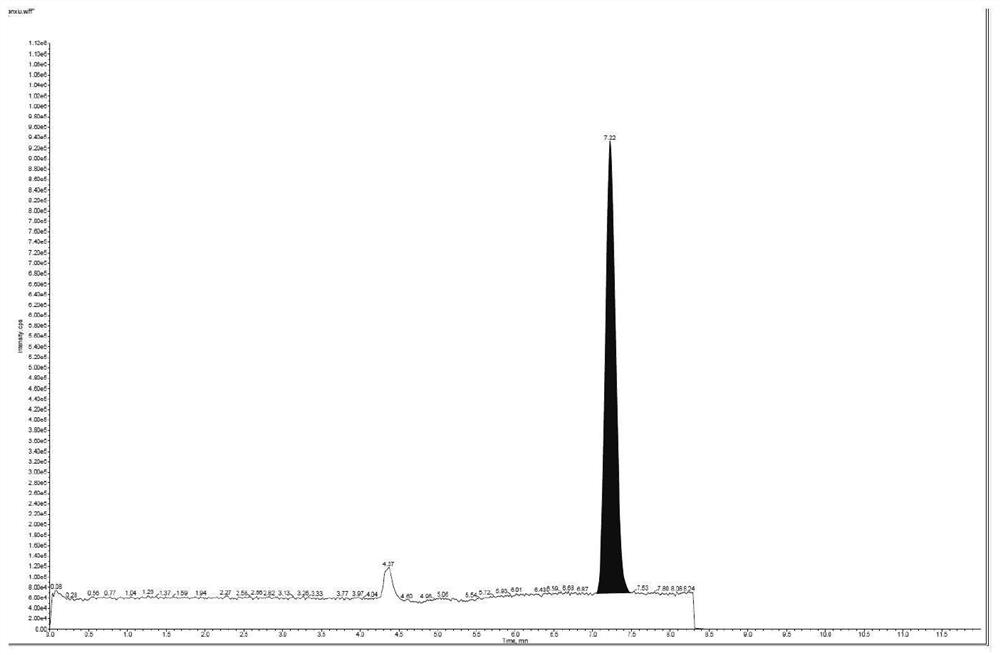
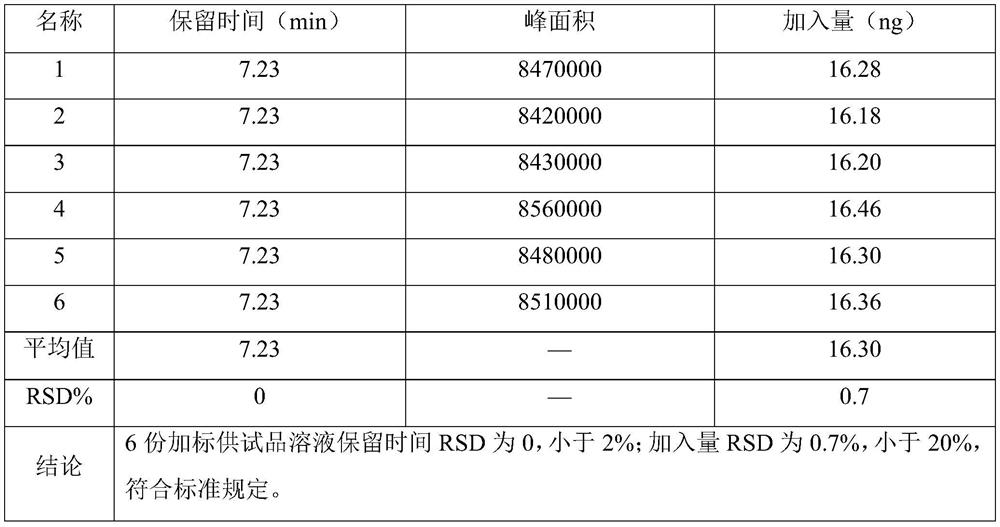
ActiveCN114354800ASolve the problem that the nature is lively and cannot be directly determined by liquid massShort reaction timeComponent separationHydrolysisCefuroximum
The invention relates to the technical field of analysis and detection, in particular to a method for analyzing the content of acetyl bromide in cefuroxime axetil. According to the analysis method, the content of acetyl bromide in cefuroxime axetil is measured by using a triple four-stage liquid chromatograph-mass spectrometer, acetyl bromide is used as a reference substance, 2, 4-dinitrophenylhydrazine is used as a derivatization reagent, and the content of acetyl bromide in cefuroxime axetil is analyzed by using a standard curve method. According to the analysis method disclosed by the invention, the problems that acetyl bromide is extremely active, easy to hydrolyze and difficult to measure are solved through a pre-column derivatization method, and the detection method is efficient and accurate.
Owner:SHANDONG UNIV +1
Synthesis method of cordycepin
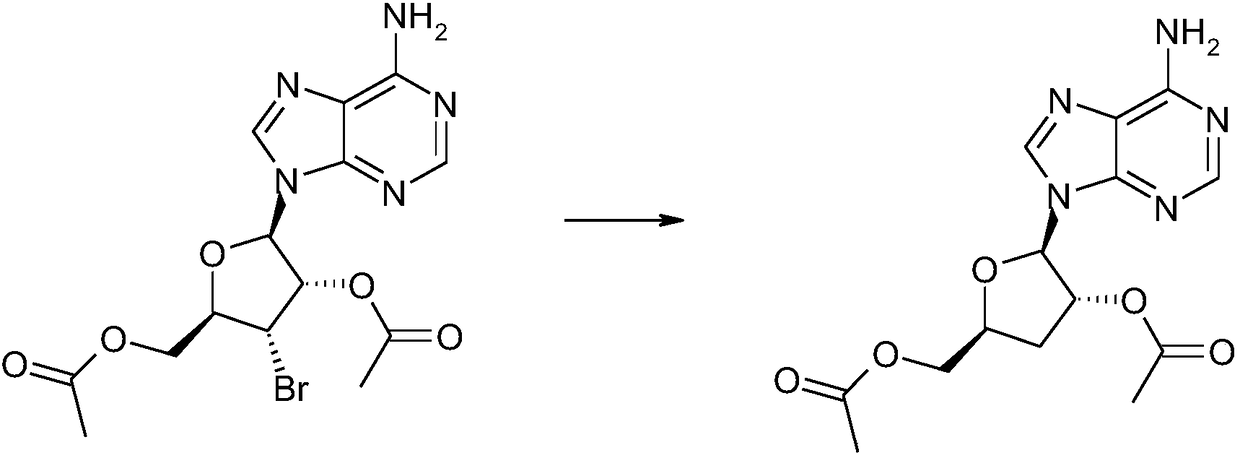
InactiveCN108864233AHigh yieldLow costSugar derivativesSugar derivatives preparationSodium bicarbonateAdenosine
The invention relates to a synthesis method of cordycepin and belongs to the technical field of cordyceps health products. The synthesis method comprises the following steps: adding adenosine, triethyl orthoformate and glacial acetic acid into a reaction flask, carrying out pressure-reduced concentration until the concentrated product is dry to obtain a caramel-like product A; dissolving the obtained caramel-like product A in dichloromethane, dropwise adding acetyl bromide at 0 DEG C, reacting for 4-6 hours under room temperature, continuously dropwise adding water after the reaction is ended,stirring for 10 minutes under room temperature, adjusting the pH value to 8 by using saturated sodium bicarbonate, and separating an organic layer to obtain a caramel-like product B; adding triethylsilicane and trifluoroacetic acid into the obtained caramel-like product B, heating and refluxing for 12 hours, carrying out pressure-reduced concentration until the concentrated product is dry, adding a saturated methanol ammonia solution, reacting under room temperature, carrying out pressure-reduced concentration until the concentrated product is dry, recrystallizing residues by using alcohol to obtain white solid which is cordycepin. The synthesis method has the advantages of high yield, low cost, low waste and simplicity and convenience in operation.
Owner:SHANDONG BENYUE BIOTECH
Papermaking black liquid lignin content detection method
ActiveCN108872119AImprove accuracyGood repeatabilityColor/spectral properties measurementsSpecific volumeAbsorbance
The invention discloses a papermaking black liquid lignin content detection method which comprises the following steps: drying papermaking black liquid lignin and grinding into fine powder to obtain alignin standard sample; taking the defined amount of the lignin standard sample, utilizing an acidized acetyl bromide method to acetylize lignin, diluting with water to a specific volume, utilizing an ultraviolet spectrophotometer to measure absorbance at the 280nm position, calculating out solution absorbance according to a formula, performing a plurality of groups of parallel experiments and taking an average value; taking the defined amount of the lignin standard sample, utilizing a KLASON method and an acid-soluble lignin detection method to detect the total content X of KLASON lignin andacid-soluble lignin and calculating out corrected absorbance; taking a lignin sample to be measured, drying, grinding into fine powder, utilizing an acidized acetyl bromide method to acetylize lignin, diluting with water to a specific volume, utilizing the ultraviolet spectrophotometer for measuring absorbance at the 280nm position and calculating out the lignin content according to the formula.When the detection method disclosed by the invention is utilized for detecting the papermaking black liquid lignin content, the detection method has the advantages of higher accuracy, good repeatability, short detection time, high efficiency and wide application range; furthermore, the detection method is suitable for detecting high-content lignin and low-content lignin.
Owner:SHANGHAI CHANGFA NEW MATERIAL CO LTD
Process for manufacture of a 4-bromo-2-oxyimino butyric acid and its derivatives
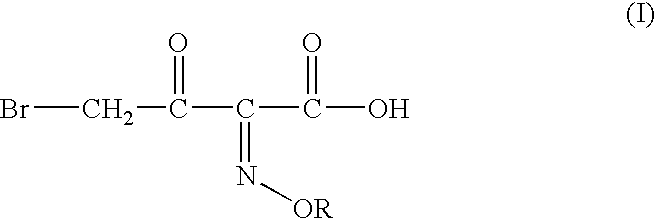
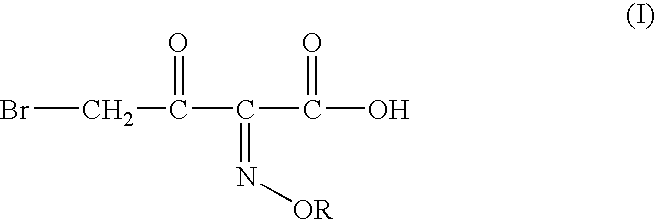
InactiveUS20040054224A1Convenient and safe and cost-effectiveUrea derivatives preparationOrganic compound preparationArylOrganic solvent
A process for producing 4-bromo-2-oxyimino butyric acid, predominantly as the (Z)-isomer of formula (I), wherein R is hydrogen; a linear or branched C1-4 alkyl group; a linear or branched C1-4 alkyl group substituted by a carboxylic acid or an aryl group; a substituted or unsubstituted cyclic alkyl group of 3-6 carbon atoms or a substituted or unsubstituted aryl group. The product is produced by reacting bromine with a 2-(oxyimino)-3-oxo butyric acid derivative of formula (II) wherein R is as defined above and R<1 >is a tert-butyl group in presence of an organic solvent and in presence of a C1-4 alcohol and acetyl bromide at a temperature ranging from about -15° C. to about +15° C. Bromine is used in a proportion of about 0.90 to about 1.35 moles per mole of compound (II), preferably 0.90 to 1.10 moles. The acetyl bromide is used in molar proportions of 0.9 to 2 moles per mole of compound (II), preferably 0.9 to 1.5 moles.
Owner:LUPIN LTD
Detection method for lignin content of papermaking pre-cooking liquor
ActiveCN108872118AImprove accuracyGood repeatabilityColor/spectral properties measurementsPapermakingAbsorbance
The invention discloses a detection method for a lignin content of papermaking pre-cooking liquor. The detection method comprises the following steps: drying lignin of papermaking pre-cooking liquor and grinding the lignin into fine powder to obtain a lignin standard sample; taking the defined amount of lignin standard sample, preparing a gradient-concentration solution, utilizing an acidizing acetyl bromide method to acetylize lignin, utilizing an ultraviolet spectrophotometer to measure absorbance at the 280nm position and making a standard curve between the absorbance and the lignin concentration; taking the defined amount of the lignin standard sample, utilizing a KLASON method and an acid-soluble lignin detection method to detect contents of KLASON lignin and acid-soluble lignin and correcting the standard curve; taking a lignin sample to be detected, drying, grinding into powder, acetylizing lignin, utilizing the ultraviolet spectrophotometer to measure absorbance at the 280nm position and calculating out the lignin content according to the corrected standard curve. The detection method disclosed by the invention can be utilized for detecting the lignin content of the papermaking pre-cooking liquor and has the advantages of higher accurate rate, good repeatability, short detection time, high efficiency, wide application range and suitability for high-content and low-content lignin detection.
Owner:SHANGHAI CHANGFA NEW MATERIAL CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com