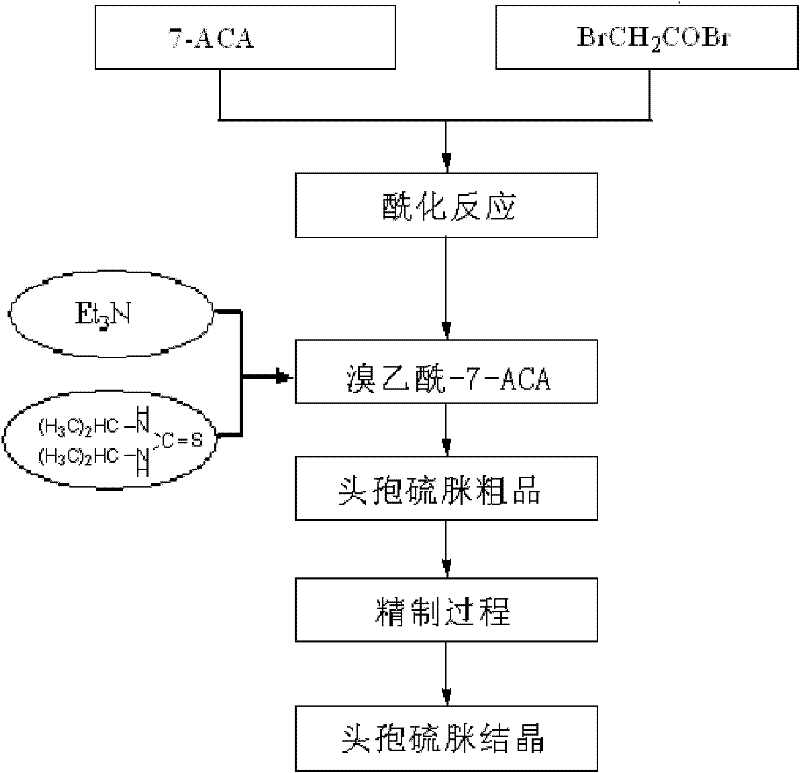
Preparation method of high-purity cefathiamidine
A technology of cefathiamidine and diisopropylthiourea, which is applied in the field of preparation of high-purity cefathiamidine, can solve problems such as thermal instability, and achieve the effects of simple and controllable operation, reduced residue, and high production yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036]At 40°C, add 10g bromoacetyl-7-ACA, 80ml dichloromethane and 8mL methanol to a 250ml three-neck flask, add triethylamine dropwise until bromoacetyl-7-ACA just dissolves; add N,N'-diiso Propylthiourea 4.5g, reacted for 3 hours. Cool down to 6-8°C, and add 100ml of acetone dropwise. After the addition, continue to stir for 30 minutes, filter with suction, wash with acetone, drain, and vacuum-dry at room temperature to obtain 9.2 g of off-white solid with a yield of 92%.
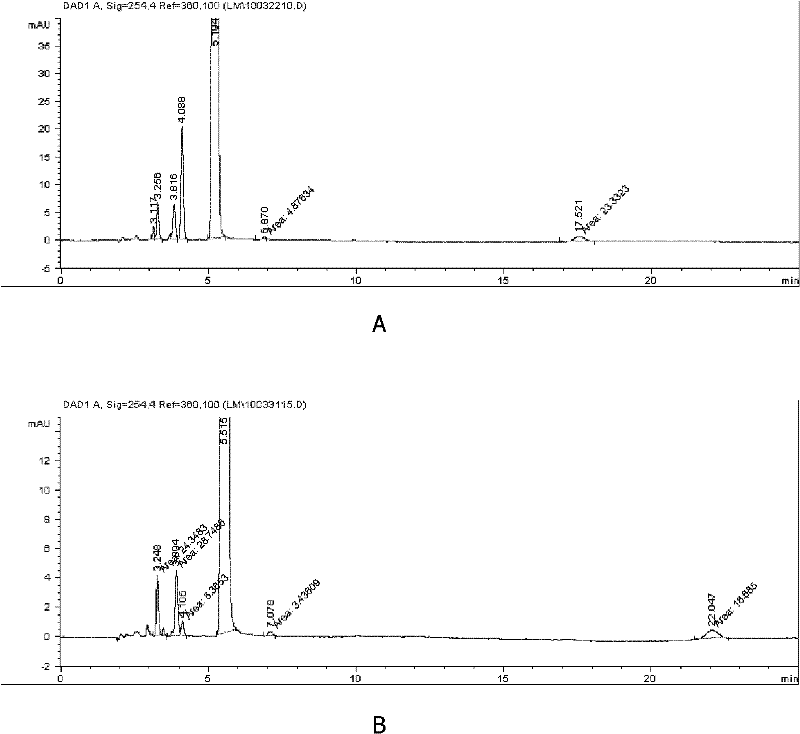
[0037] Adopt high performance liquid chromatography instrument, according to the chromatographic condition of relevant impurity item method in 2010 edition " Chinese Pharmacopoeia " cefathiamidine, the cefathiamidine that makes is carried out HPLC analysis, collection of illustrative plates is as follows figure 2 As shown in B, the peak at 5.5 minutes represents cefathiamidine, and the peak at 4.1 minutes represents bromoacetyl-7-ACA. Calculated according to the area percentage, bromoacetyl-7-ACA accoun...
Embodiment 2
[0044] At 25°C, add 10g bromoacetyl-7-ACA, 80ml dichloromethane and 5mL methanol to a 250ml three-neck flask, add triethylamine dropwise until bromoacetyl-7-ACA just dissolves; add N,N'-diiso Propylthiourea 4.2g, reacted for 4 hours. The temperature was lowered to 0-3°C, and 80 ml of dichloromethane was added dropwise. After the addition, continue to stir for 30 minutes, filter with suction, wash with acetone, drain, and dry in vacuum at room temperature to obtain 8.5 g of white solid, with a yield of 85%.
[0045] Using a high-performance liquid chromatography instrument, according to the chromatographic conditions of the method for the impurity item in the 2010 edition of "Chinese Pharmacopoeia" cefathiamidine, the prepared cefathiamidine was analyzed by HPLC (the map is omitted), calculated according to the area percentage, bromoacetyl-7 -ACA accounts for 0.20%, and the purity of cefathiamidine is 98.31%.
Embodiment 3
[0047] At 30°C, add 10g bromoacetyl-7-ACA, 110ml dichloromethane and 10mL ethanol to a 250ml three-neck flask, add triethylamine dropwise until bromoacetyl-7-ACA just dissolves; add N,N'-diiso Propylthiourea 4.3g, reacted for 4 hours. Cool down to 3-6°C, and add 80ml of acetone dropwise. After the addition, continue to stir for 30 minutes, filter with suction, wash with acetone, drain, and dry in vacuum at room temperature to obtain 9.0 g of white solid with a yield of 90%.
[0048] Using a high-performance liquid chromatography instrument, according to the chromatographic conditions of the method for the impurity item in the 2010 edition of "Chinese Pharmacopoeia" cefathiamidine, the prepared cefathiamidine was analyzed by HPLC (the map is omitted), calculated according to the area percentage, bromoacetyl-7 -ACA accounts for 0.18%, and the purity of cefathiamidine is 98.20%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com