Preparation method of ulipristal acetate bulk drug impurities
A technology of ulipristal acetate and raw materials, applied in the direction of steroids, organic chemistry, etc., can solve problems such as not involved, and achieve the effects of enhanced control, easy availability of raw materials, and easy control of reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
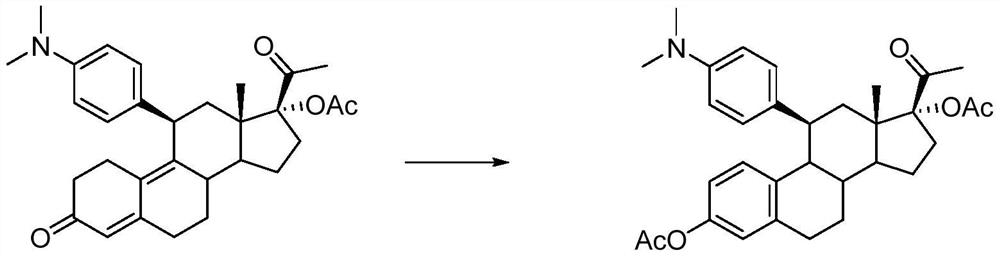
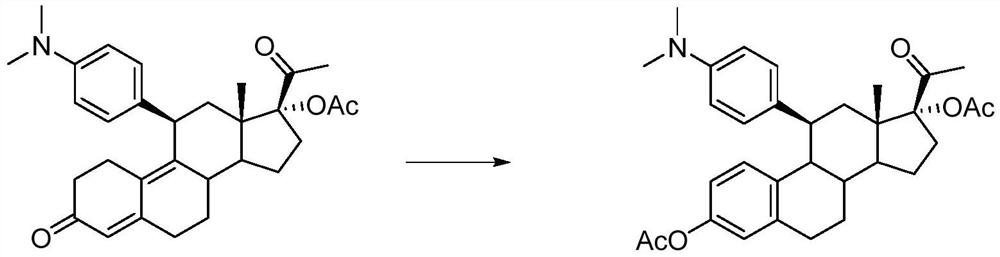
[0023] Under nitrogen protection, add 5g (0.01mol) of ulipristal acetate and 25ml of dichloromethane into a 100ml three-necked flask, stir to dissolve, add 1ml (0.01mol) of acetic anhydride, 2.4ml (0.032mol) of acetyl bromide, and React for 6 hours, pour into 100ml of water, add saturated sodium bicarbonate solution to react excess acetic anhydride and acetyl bromide, let stand to separate layers, extract the aqueous layer with dichloromethane, wash the organic phase with water, and evaporate to dryness to obtain an oily substance. The oily product was purified by column chromatography to obtain 4.6 g of off-white powdery solid with an HPLC purity of 97.6%. HR-ESI-Ms (M+H): 518.3. H-NMR (CDCl 3 ,400MHz)δ:0.87(s,3H,C 18 -Me),1.96(s,3H,C 21 -Me),2.11(s,3H,C 17 -Ac),2.27(s,3H,C 3 -Ac),3.06(s,6H,-N(CH 3 ) 3 ),
[0024] 1.09~2.35(m,10H), 2.73~3.19(m,4H), 6.55~6.67(m,3H, Benzene Ring), 6.76~6.81(m,4H, Benzene Ring).
Embodiment 2
[0026] Under nitrogen protection, add 5g (0.01mol) of ulipristal acetate and 25ml of dichloromethane into a 100ml three-necked flask, stir to dissolve, add 1ml (0.01mol) of acetic anhydride, 2.4ml (0.032mol) of acetyl bromide, and React for 6 hours, pour into 100ml of water, add saturated sodium bicarbonate solution to react excess acetic anhydride and acetyl bromide, let stand to separate layers, extract the aqueous layer with dichloromethane, wash the organic phase with water, and evaporate to dryness to obtain an oily substance. The oil was recrystallized from isopropyl ether to obtain 4.3 g of off-white powdery solid with an HPLC purity of 95.9%.
Embodiment 3
[0028] Under nitrogen protection, add 5 g (0.01 mol) of ulipristal acetate and 50 ml of dichloromethane into a 100 ml three-neck flask, stir to dissolve, add 1 ml (0.01 mol) of acetic anhydride, 2.5 ml (0.034 mol) of acetyl bromide, and React for 6 hours, pour into 100ml of water, add saturated sodium bicarbonate solution to react excess acetic anhydride and acetyl bromide, let stand to separate layers, extract the aqueous layer with dichloromethane, wash the organic phase with water, and evaporate to dryness to obtain an oily substance. The oil was recrystallized from isopropyl ether to obtain 4.5 g of off-white powdery solid with an HPLC purity of 96.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com