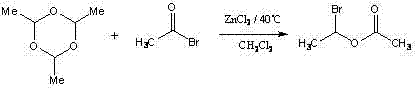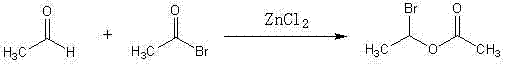Method for synthesizing (R,S)1-bromoethyl acetate
A technology of ethyl acetate and bromination, applied in the field of cefuroxime axetil key intermediate (R field, can solve the problems of increasing the amount of acidic waste water, increasing the three wastes, complicated operations, etc., reducing the discharge of the three wastes, facilitating control, Simple to use effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Add 45 ml of N,N-dimethylacetamide to a 250 ml four-necked flask equipped with a stirrer and a thermometer, cool to -17~-15°C, add 10.4 ml (0.185 mol) of acetaldehyde while stirring. Add 0.3g (0.002 mol) of 1-tetralone, cool the temperature of the material to -20~-18°C, and slowly drop in 21.94g (0.178 mol) of acetyl bromide. Control the temperature at 4-6°C and fully stir the reaction for 7 hours. The gas phase content is 94.16%, and the α-bromine impurity content is 0.76%. The molar yield is 89.65%.
Embodiment 2
[0033] Add 45 ml of acetonitrile to a 250 ml four-necked flask equipped with a stirrer and a thermometer, cool to -17~-15°C, add 10.4 ml (0.185 mol) of acetaldehyde while stirring and keeping warm. Add 0.1g (0.00068 mol) of 1-tetralone, cool the temperature of the material to -20~-18°C, and slowly drop in 21.94g (0.178 mol) of acetyl bromide. Control the temperature at -10°C and fully stir the reaction for 12 hours. The gas phase content is 94.34%, and the α-bromine impurity content is 0.11%. The molar yield is 75.71%.
Embodiment 3
[0035] Add 45 ml of dimethyl sulfoxide to a 250 ml four-necked flask equipped with a stirrer and a thermometer, cool to -17~-15°C, add 10.4 ml (0.185 mol) of acetaldehyde while stirring. Add 0.026g (0.000178 mol) of 1-tetralone, cool the temperature of the material to -20~-18°C, and slowly drop in 21.94g (0.178 mol) of acetyl bromide. The temperature was controlled at 25°C and the reaction was sufficiently stirred for 2 hours. The gas phase content is 85.98%, and the α-bromine impurity content is 3.51%. The molar yield is 78.49%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com