Method for ultrasonically synthesizing glycal
A technology of ultrasonic wave and alkenose, applied in the fields of medicinal chemistry and organic synthesis, can solve the problems of separation and purification, low yield, high cost, complicated intermediate product processing, etc., and achieves the effect of reducing cost and thorough reaction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
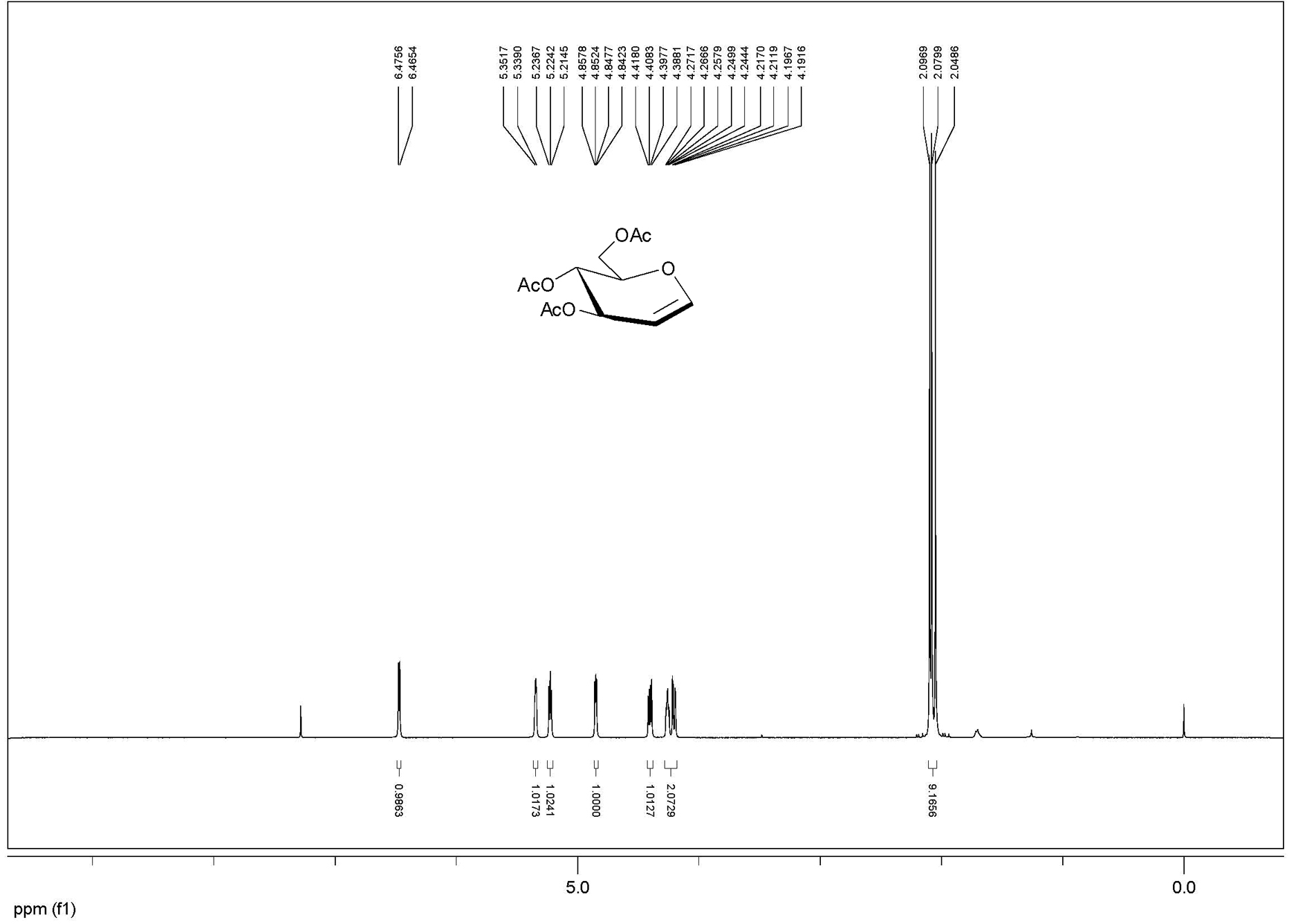
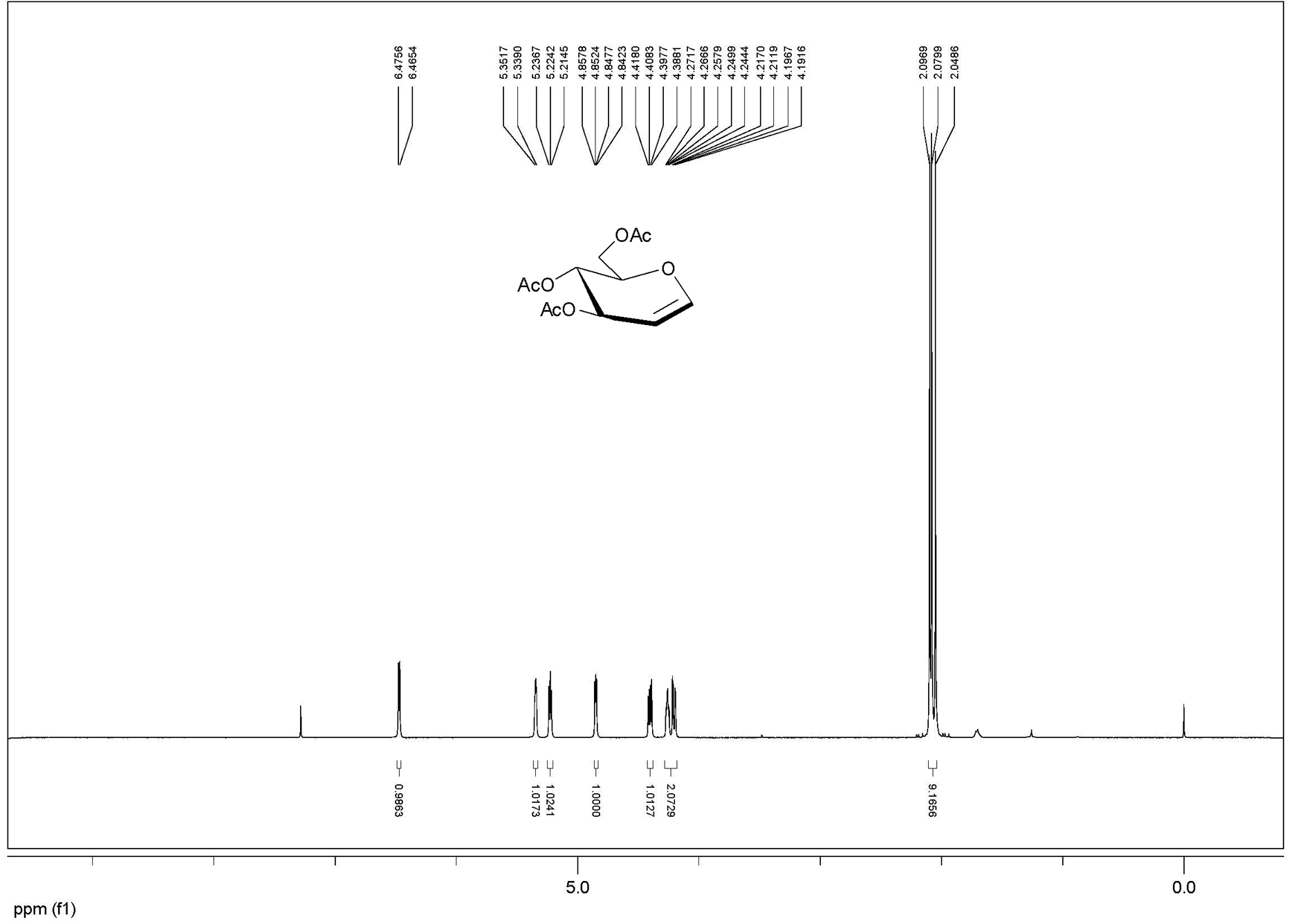
[0028] Example 1: 10 g of glucose, 100 mL of acetic acid, 32.5 mL of acetic anhydride, 0.5 g of p-toluenesulfonic acid were added to a 500 mL round bottom flask, and ultrasonic reaction was performed for 10 minutes, and 12.5 mL of acetyl bromide, methanol 9.0 mL, ultrasonic oscillation, power 420W (model DST20500) and react for 1 hour. TLC detected that the reaction was complete, diluted with dichloromethane, washed with water, and recrystallized with ethanol to obtain 21.9 g of bromoglucose crystals with a yield of 96%.
[0029] Add 44 g of β-cyclodextrin, 220 mL of water and 11 g of zinc powder to the above-mentioned bromosugar, ultrasonic power 480W, (model DST20500) shake for 10 minutes, add ethyl acetate to dilute, filter, and the filtrate is washed with ethyl acetate Extracted 3 times, combined the organic phases, dried with anhydrous sodium sulfate, filtered, and concentrated under reduced pressure to obtain crude acetylated glucal, and then recrystallized with ethyl ace...
example 2
[0030] Example 2: Add 10 g of glucose, 100 mL of acetic acid, 32.5 mL of acetic anhydride, 5 drops of perchloric acid in a 500 mL round bottom flask, and ultrasonically react for 5 minutes. Add 12.5 mL of acetyl bromide and 9.0 mL of methanol to the above reaction solution, and ultrasonically (Power 450W, Model DST20500) Shake and react for 1 hour, TLC detects that the reaction is complete, dilute with dichloromethane, wash with water, and recrystallize with ethanol to obtain 21.0 g of bromosugar crystals.
[0031]Add 42 g of β-cyclodextrin, 210 mL of water and 11 g of zinc powder to the above-mentioned bromosugar, ultrasonic power 450W, (model DST20500) shake and react for 20 minutes, add ethyl acetate to dilute, filter, and the filtrate is washed with ethyl acetate Extracted 3 times, combined the organic phases, dried with anhydrous sodium sulfate, filtered, and concentrated under reduced pressure to obtain crude acetylated glucal, and then recrystallized with ethyl acetate a...
example 3
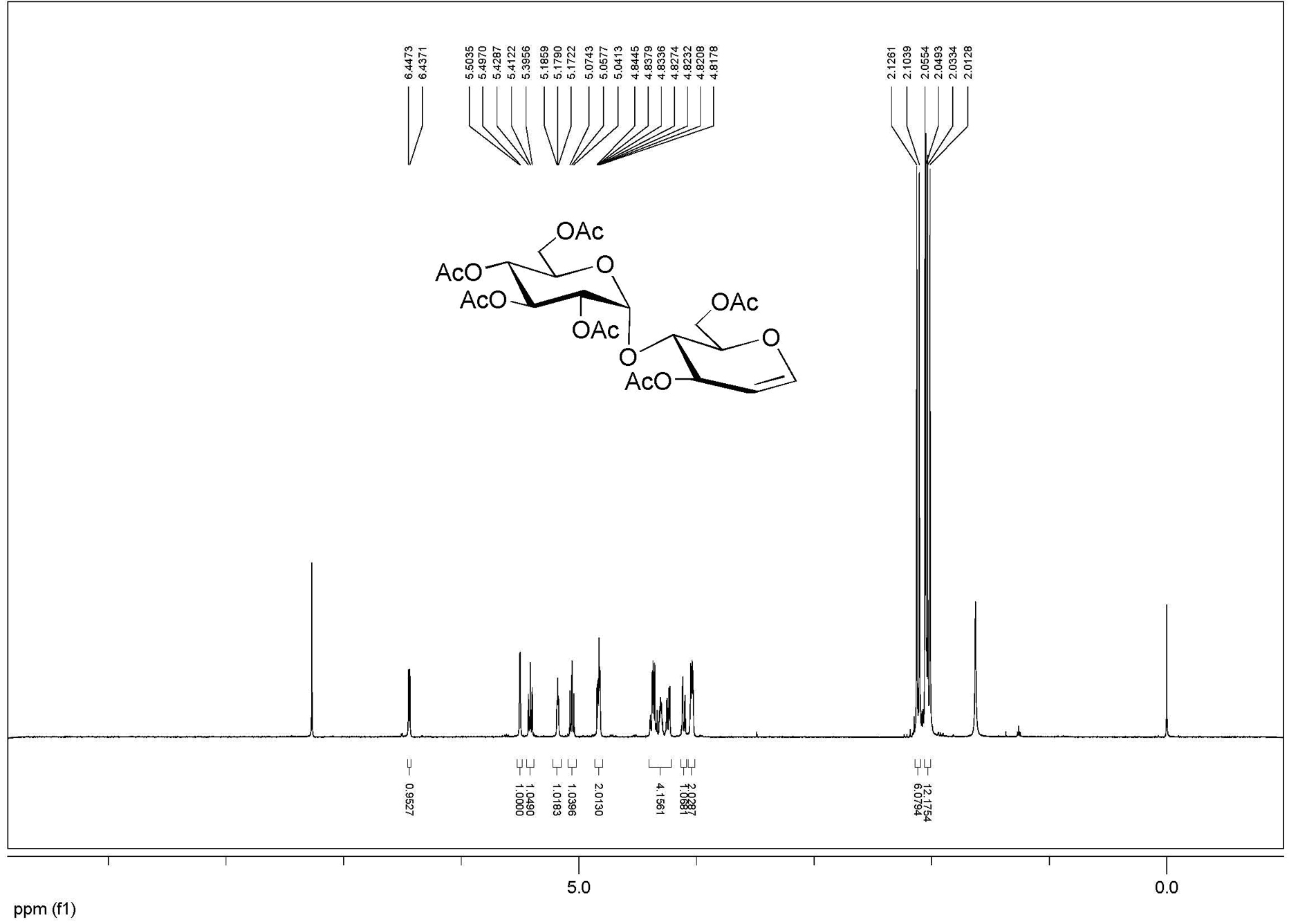
[0032] Example 3: Add 10 g of maltose, 100 mL of acetic acid, 32.5 mL of acetic anhydride, 0.5 g of p-toluenesulfonic acid into a 500 mL round bottom flask, and ultrasonically react for 20 minutes. Add 7.5 mL of acetyl bromide and 4.5 mL of methanol to the above reaction solution mL, ultrasonic power 420W, (model DST20500) shaking reaction for 1.5 hours, TLC detection of complete reaction, diluted with dichloromethane, washed with water, and recrystallized with ethanol to obtain 19.4 g of bromomaltose crystals, with a yield of 95%.
[0033] Add 40 g of β-cyclodextrin, 200 mL of water and 6 g of zinc powder to the above bromosugar, ultrasonic power 450W, (model DST20500) shake reaction for 1 hour, add ethyl acetate to dilute, filter, and the filtrate is washed with ethyl acetate Extract 3 times, combine the organic phases, add anhydrous sodium sulfate to dry, filter, and concentrate under reduced pressure to obtain crude acetylated glucal, and then recrystallize with ethyl aceta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com