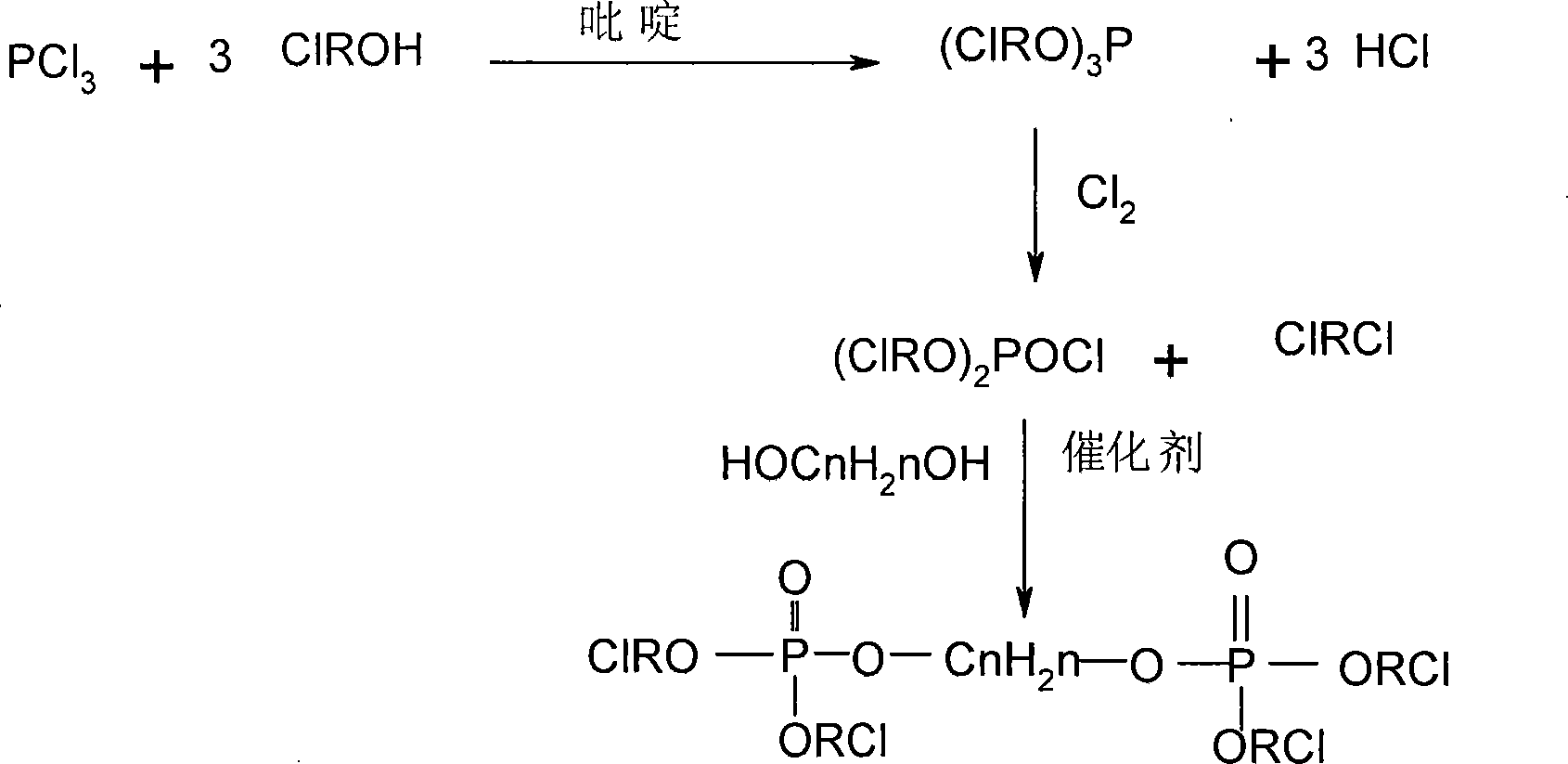
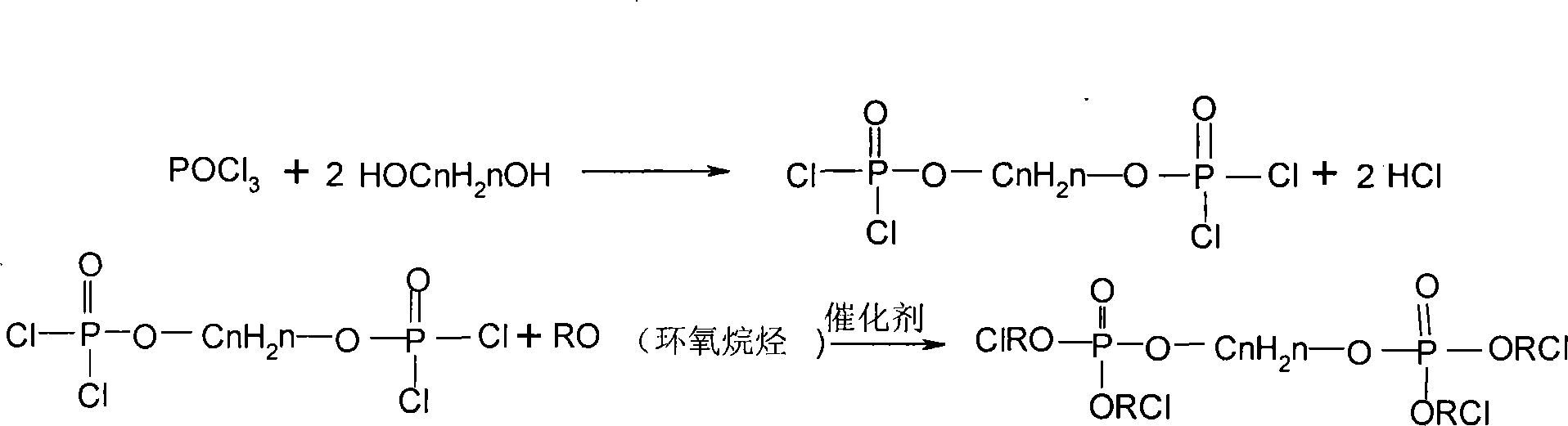
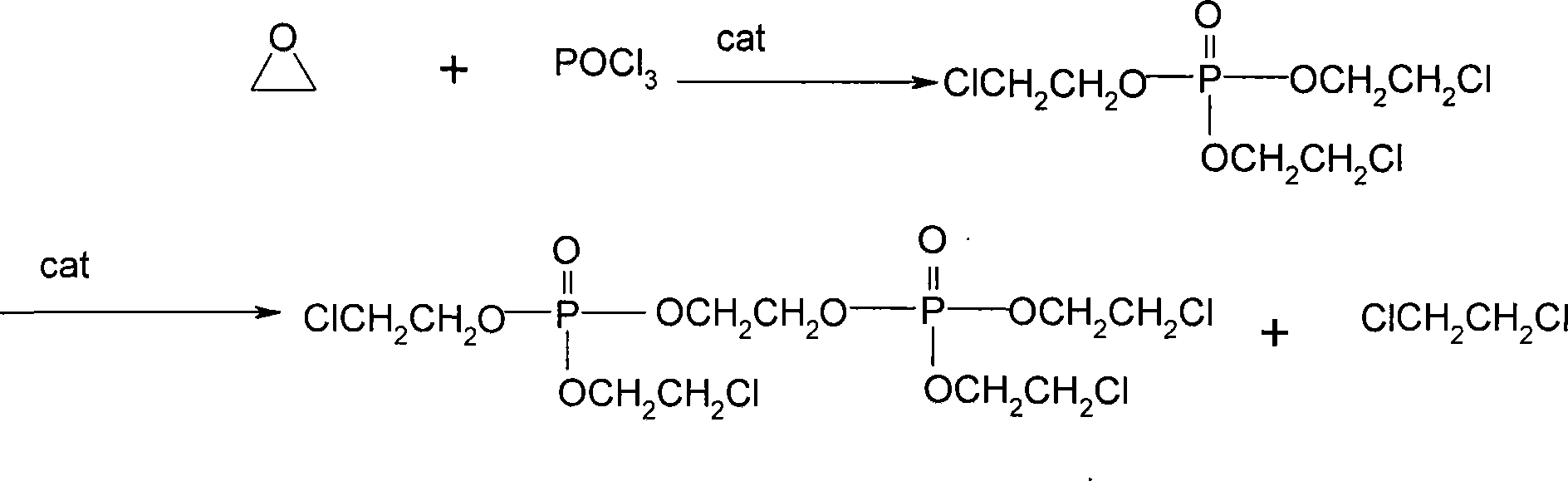
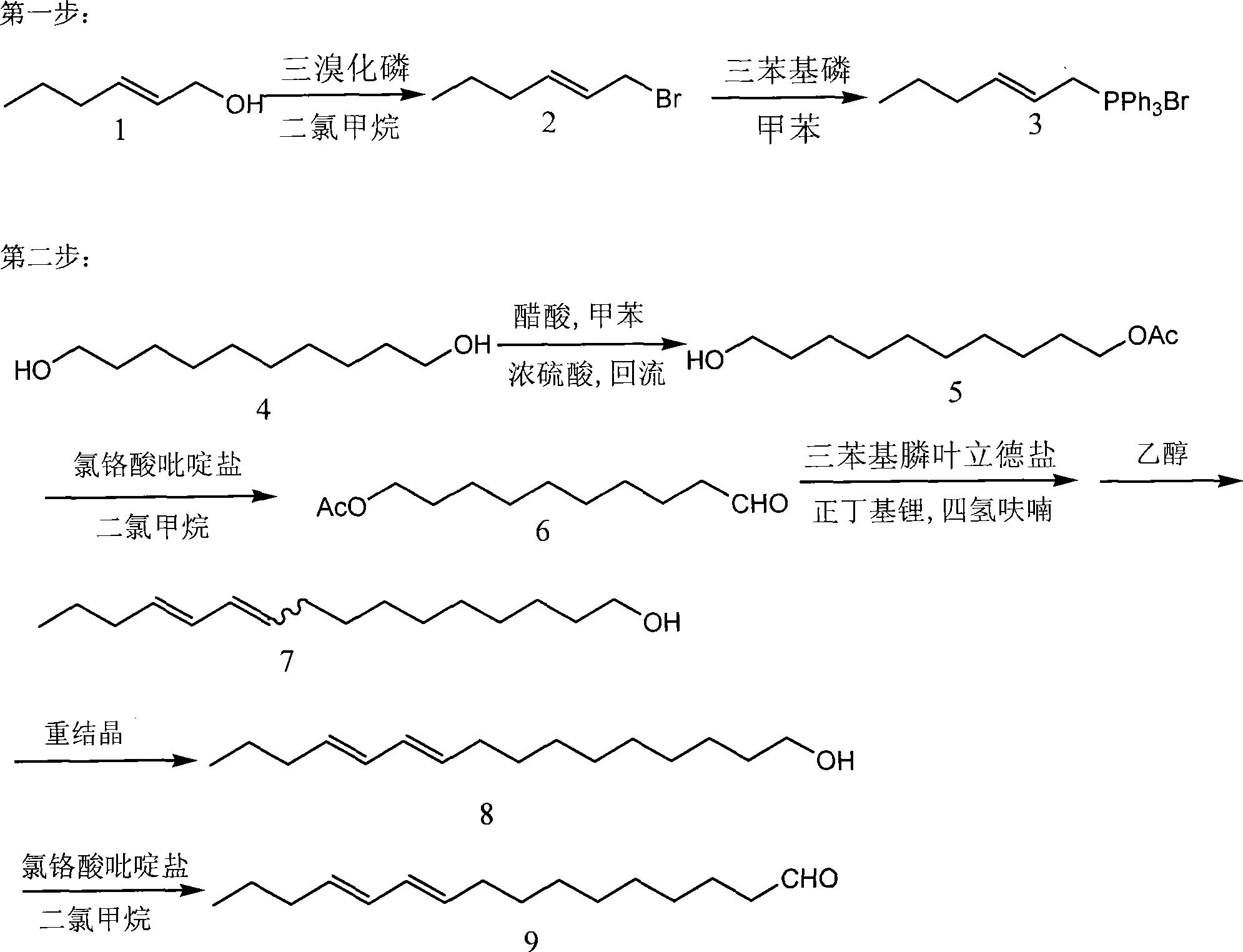
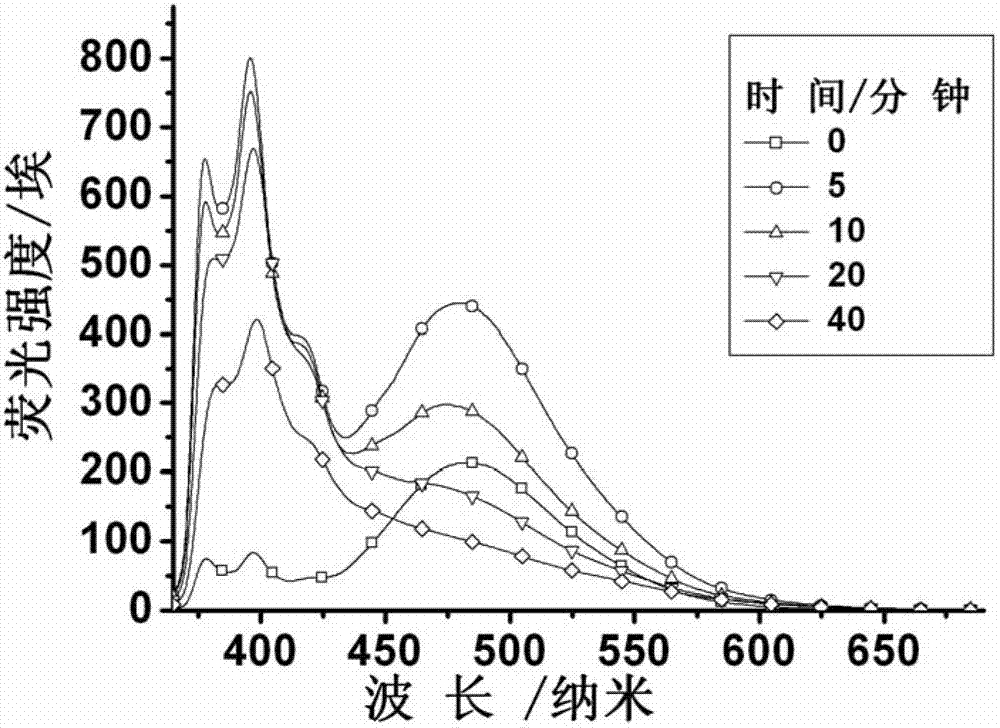
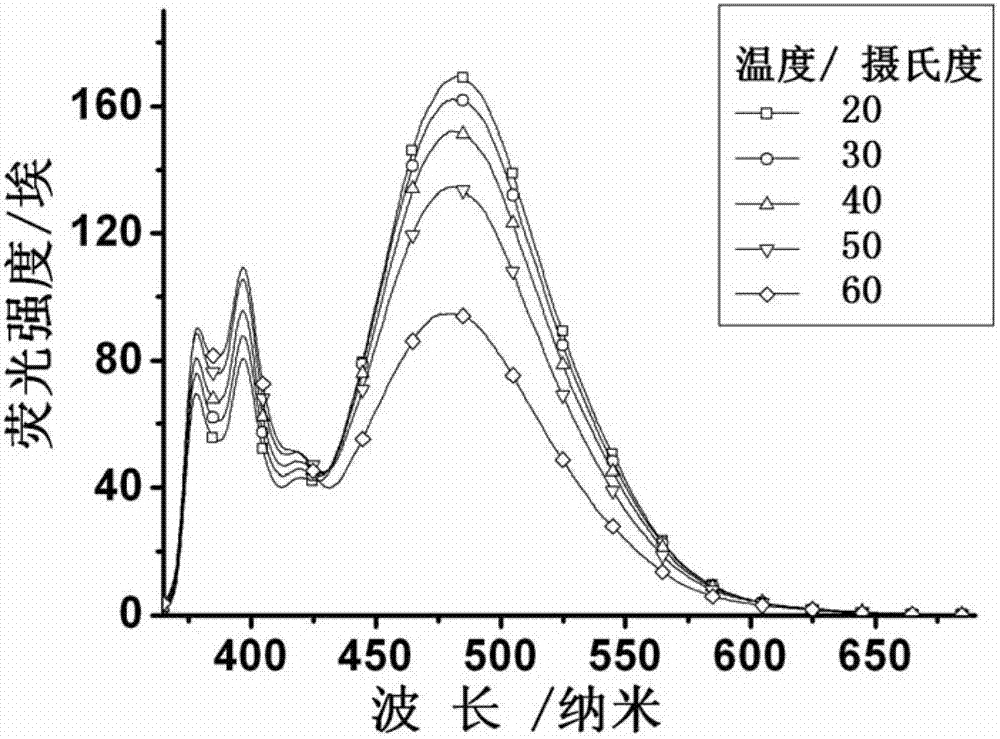
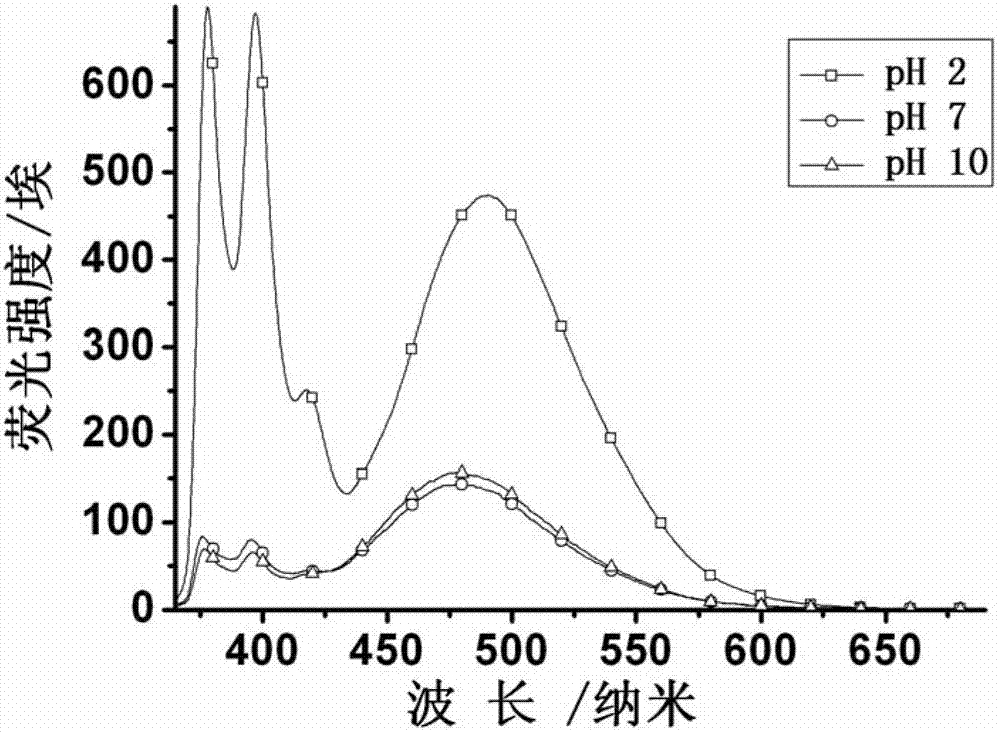
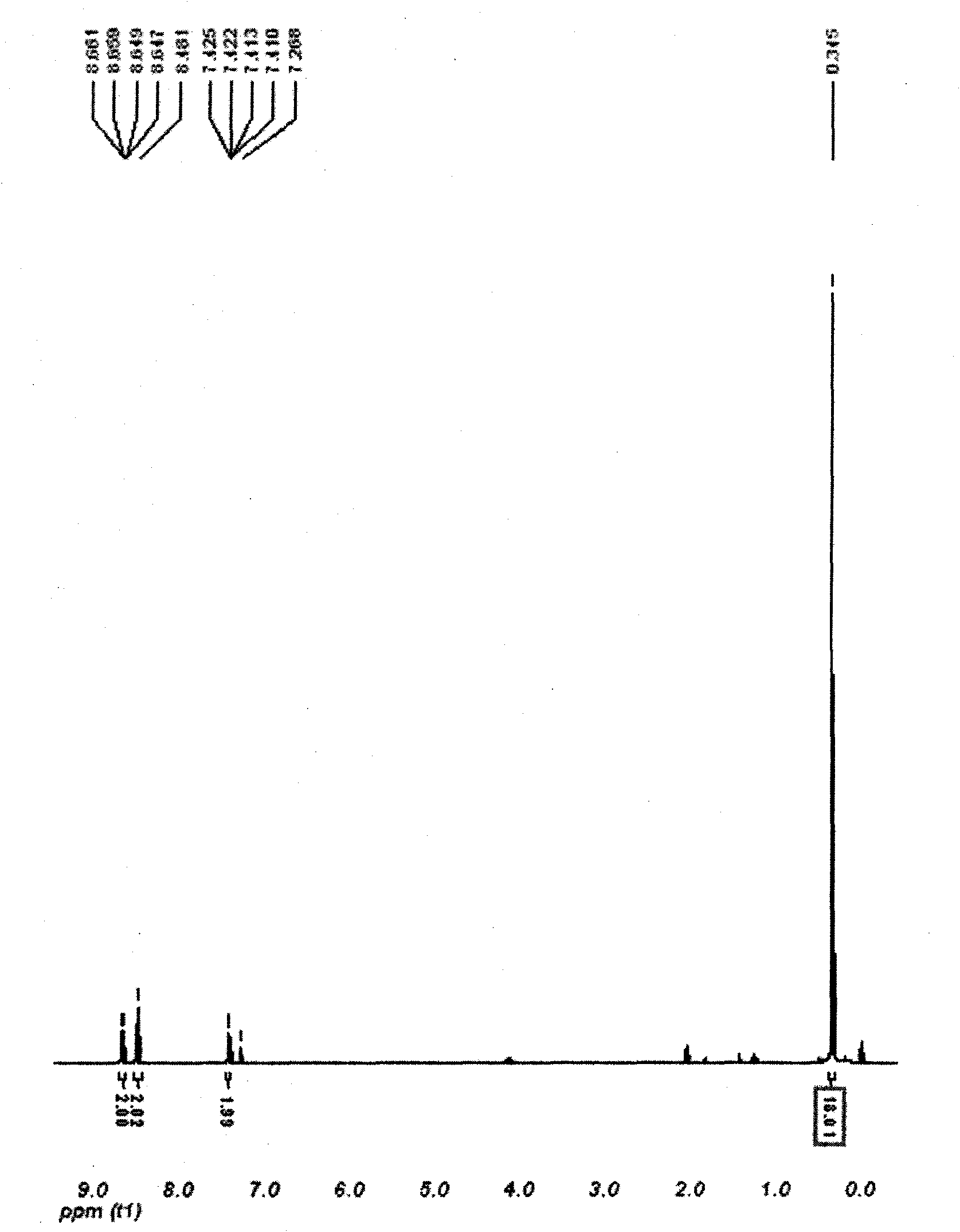
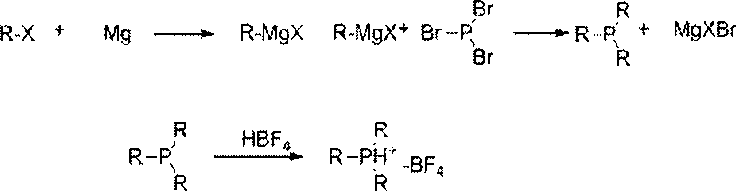Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
90 results about "Phosphorus tribromide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
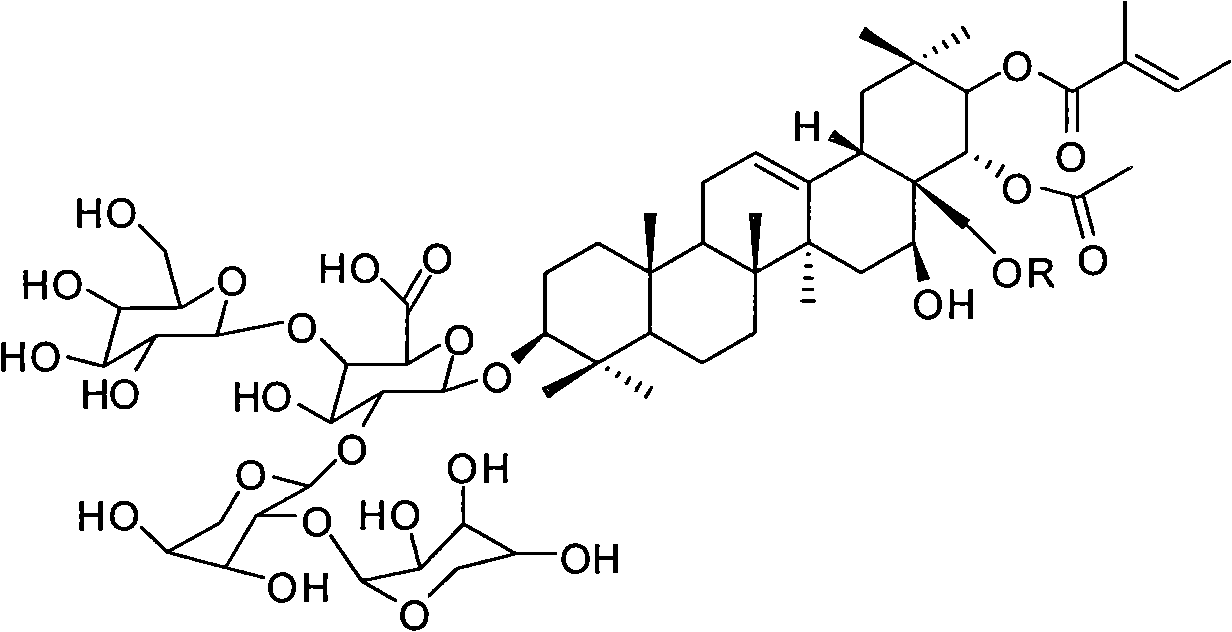
Phosphorus tribromide is a colourless liquid with the formula PBr₃. It is a colourless liquid that fumes in moist air due to hydrolysis and has a penetrating odour. It is used in the laboratory for the conversion of alcohols to alkyl bromides.
Theasaponin derivative as well as preparation method and application thereof
InactiveCN102030803AStrong foaming powerGood foam stabilityBiocideNon-ionic surface-active compoundsPhosphorus tribromideSugar
The invention relates to a theasaponin derivative as well as a preparation method and application thereof. The theasaponin derivative has a structure shown in the formula I and is prepared by the following steps of: (1) reacting triphenylchloromethane with theasaponin, and then adding bromobenzyl to react with the theasaponin; (2) reacting phosphorus tribromide with sugar, and then reacting with the bromobenzyl; (3) mixing the theasaponin obtained in the step (1) with the sugar obtained in the step (2), and then adding tritylation deprotective agents to react; and then adding phenmethyl deprotective agents in the presence of catalysts to obtain the theasaponin derivative shown in the formula I after the reaction. The preparation method has the advantages that the process is simple, the reaction conditions are easy to control, and the preparation method can be used for the commercial production. The prepared theasaponin derivative can be used as a surface active agent, the foaming power and the foam stabilization are stronger in high-salt and high-temperature environments, and the problem of poor foam stabilization in the high-salt and high-temperature environments of the traditional theasaponin is solved.
Owner:SOUTH CHINA UNIV OF TECH
Bromotetraacetylglucose, synthetic method and use thereof
ActiveCN101671375AThe method is simple and efficientHigh yieldEsterified saccharide compoundsSugar derivativesAcetylationGlucose polymers
The invention relates to a synthetic method of bromotetraacetylglucose, bromotetraacetylglucose and use thereof. The invention comprises the steps of taking glucose as raw materials, carrying out acetylation reaction by acetyl oxide as an acylating agent, then carrying out bromination reaction by phosphorous tribromide and hydrobromic acid as a brominating agent, and then carrying out recrystallization by diethyl, and at last obtaining bromotetraacetylglucose. The invention has the advantages of simple and efficient method, high yield and high purity reaching above 98.9%.
Owner:YANCHENG CITY CHUNZHU AROMA
Synthetic method for glufosinate ammonium
InactiveCN109232644AMild reaction conditionsEasy to operateGroup 5/15 element organic compoundsPhosphorus tribromideDistillation
The invention relates to a synthetic method for glufosinate ammonium. The synthetic method comprises the following steps: single-bromine substitution, amination, amino protection, chlorination ring opening, Arbuzov reaction and acidizing hydrolysis ammoniation; the single-bromine substitution means triggering alpha-site single-bromine substitution betweengamma-butyrolactone I and bromine under theexistence of catalyst and performing reduced pressure distillation, thereby acquiring a pure intermediate II alpha-bromine-gamma-butyrolactone; phosphorus tribromide is served as the catalyst; amination means triggering amination reaction between alpha-bromine-gamma-butyrolactone II and ammonium hydroxide, and then adding hydrochloric acid and reflowing, thereby acquiring an intermediate IIIalpha-amino-gamma-butyrolactone hydrochloride. The invention has the beneficial effects: 1) low-costgamma-butyrolactone is taken as a raw material, is subjected to single-bromine substitution with bromineand then is subjected to amination reaction with ammonium hydroxide; the adopted raw materials are low-cost and easily acquired; reaction conditions are mild; operation is simple and convenient; safety is high; amplifying production is feasible; reaction yield is high; product purity is high; cost is greatly lowered; the synthetic method is suitable for industrial production.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
Halogen-containing diphosphate or preparation method of halogen-containing diphosphorous acid ester
InactiveCN101215295ASimple processShorten the timePhosphorus organic compoundsPhosphorous acidPhosphorus tribromide
The invention relates to a process for preparation which contains halogen two-phosphate or halogen diphosphate, which is divided into the procedures that firstly, ethylene oxide and propylene oxide or epichlorohydrin and phosphorus oxychloride or tribromide oxygen phosphorus are reacted under the conditions of catalyst and heating and generate trialkyl phosphate with halogen, or ethylene oxide and propylene oxide or epichlorohydrin and phosphorus trichloride or phosphorus tribromide are reacted under the conditions of catalyst and heating and generate phosphorous acid, secondly, the trialkyl phosphate with halogen conducts transesterification under the conditions of catalyst and heating to gain diphosphate with halogen and halogenated hydrocarbons, or phosphate-containing brine conducts transesterification under the same condition to gain diphosphite with halogen and halogenated hydrocarbons. The catalyst of the first procedure adopts acid catalyst, and the catalyst of the second procedure adopts alkaline catalyst, and the dosage of catalyst of each procedure is respectively 0.05-2.5 of the total amount of reagent raw material of the procedure. The total yield of the product of the invention is 90-97%, and the product has the advantages of good color, low acid value, excellent viscosity, and does not need additional decolor process.
Owner:ZHENJIANG SANWA FLAME RETARDANT ENG TECH CO LTD
Method for synthesizing compound E10, E12-hexadecadienal in sex pheromone of legume pod borer
ActiveCN101434521AIncrease profitIncrease contentCarbonyl compound preparation by oxidationMarucaPhosphorus tribromide
A synthetic method of compound E10, E12-hiago dienal in maruca testulalis geyer sex pheromone is characterized in that the compound adopts trans-2-hexenol and 1, 10-decanediol as the substrate; after halogenating reaction, bromide can be obtained from the trans-2-hexenol which is then reacted with phosphorus tribromide to obtain triphenylphosphine ylide salt; after the esterification reaction of the 1, 10-decanediol, the 1, 10-decanediol protected by acetic ester can be obtained, and then after oxidation reaction, 10-oxo decyl acetate can be obtained; after Witting reaction and then quenching reaction of the triphenylphosphine ylide salt and the 10-oxo decyl acetate, 10, 12-hiago dienal can obtained by separation; after the process of recrystal, trans 10, 12-hiago dienal with transverse structure can be obtained, and then after the oxidation reaction, E10, E12-hiago dienal is obtained. The method of the invention adopts materials which are cheap and easy to be obtained and uses simple and reasonable syntheticroute, the operation is simple and safe, the utilization rate of the materials is high and the main ingredient compound of the maruca testulalils geyer sex pheromone can be synthesized with low cost in a short time.
Owner:宁波纽康生物技术有限公司
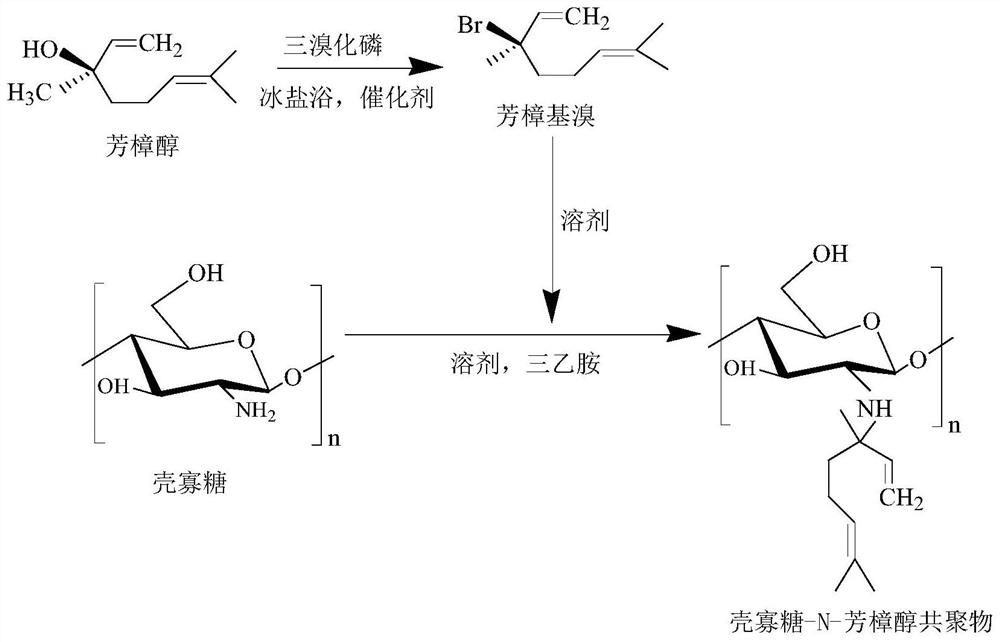
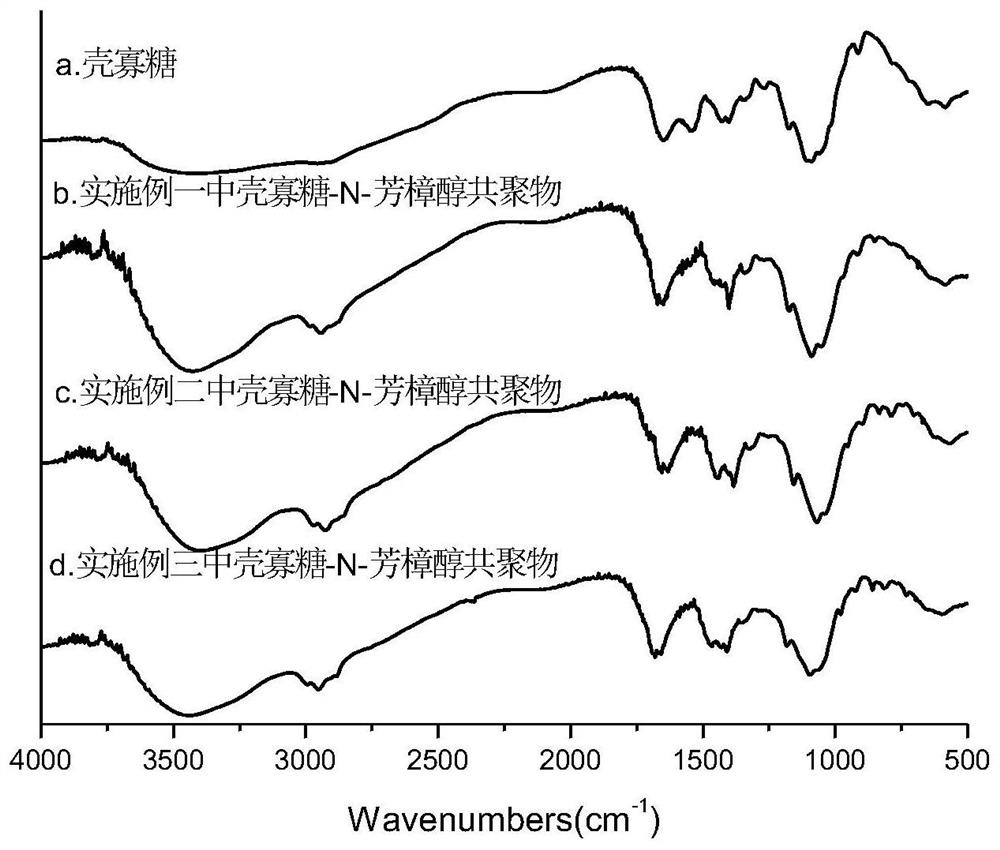
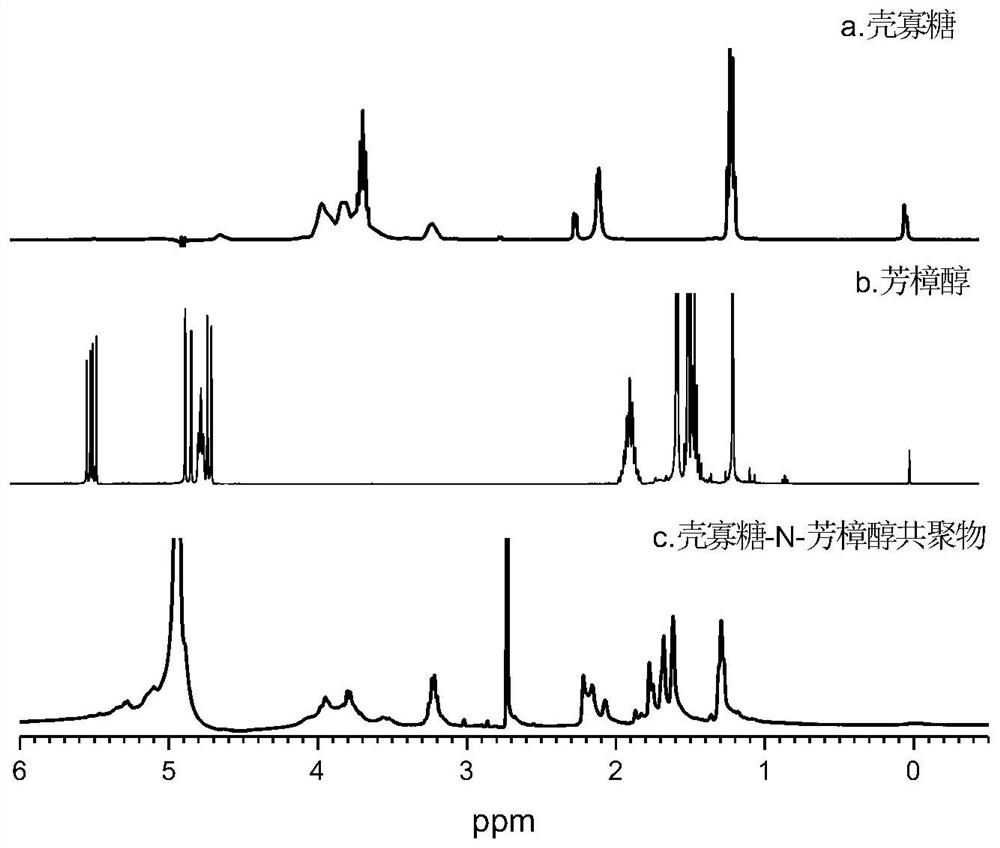
Chitosan oligosaccharide-N-linalool copolymer and preparation method and application thereof
ActiveCN111620966AGood anti-inflammatory activityEasy to makeAntibacterial agentsBiocidePhosphorus tribromideLinalool
The invention discloses a chitosan oligosaccharide-N-linalool copolymer and a preparation method and application thereof. The preparation method of the copolymer comprises the following steps: (1) performing bromination reaction on linalool and phosphorus tribromide at a certain temperature to obtain faint yellow oily liquid linaloyl bromide; and (2) mixing and reacting a certain amount of linaloyl bromide and chitosan oligosaccharide, and treating to obtain the chitosan oligosaccharide-N-linalool copolymer. The alkylation reaction is performed on linalool with anti-inflammatory activity and C2-site active amino of chitosan oligosaccharide, the preparation process and the purification process are simple, and the obtained chitosan oligosaccharide-N-linalool copolymer has good water solubility, thermal stability and anti-inflammatory activity and has good application prospects in the fields of food, medicine, cosmetics, agriculture and the like.
Owner:JIANGNAN UNIV
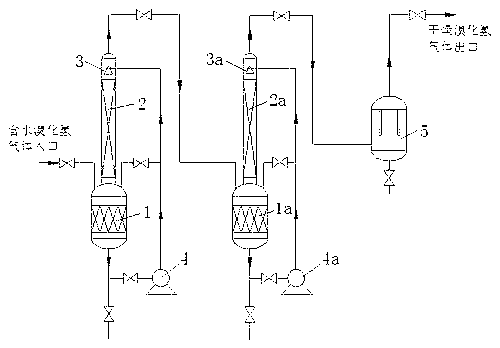
Hydrogen bromide gas drying method
ActiveCN103318843ARemove applicableImprove dehydration efficiencyBromine/hydrogen-bromidePhosphorus tribromideLiquid state
The invention relates to a hydrogen bromide gas drying method, and belongs to the field of inorganic chemical engineering and hydrogen bromide gas purification. According to the method, hydrogen bromide gas with water content enters a filling material drying tower from the bottom; a liquid-state inorganic bromide phosphorus tribromide is delivered from the top of the filling material drying tower; the phosphorus tribromide liquid and the hydrogen bromide gas are subjected to countercurrent contact in the filling material drying tower; drying is carried out by using a two-stage series filling material drying tower; the gas is processed by using a defogger, such that dried hydrogen bromide gas with a water content lower than 20ppm is obtained, and bromine element content in the hydrogen bromide gas is partially removed. The phosphorus tribromide liquid can be circularly used. When the concentration of the phosphorus tribromide liquid is lower than 80%, the phosphorus tribromide liquid can be purified through vacuum distillation, and can be recovered. Compared with current other methods, such the method provided by the invention, dehydration efficiency is high, and hydrogen bromide gas water content can be lower than 20ppm. The method is suitable to be used in a production process with relatively low hydrogen bromide gas pressure, and is especially suitable to be used in water removing of hydrogen bromide gas synthesized by combustion bromine in hydrogen.
Owner:中昊光明化工研究设计院有限公司
Total synthesis method of trans-resveratrol
InactiveCN103570508AWide variety of sourcesMild reaction conditionsOrganic compound preparationCarboxylic acid esters preparationBenzoic acidBenzoyl bromide
The invention discloses a total synthesis method of trans-resveratrol. The total synthesis method comprises the following steps: (1) enabling 3,5-dihydroxy-benzoic acid to react with dimethyl sulfate, so as to generate 3,5-dimethoxy methyl benzoate; (2) enabling the 3,5-dimethoxy methyl benzoate to react with a reducing agent, so as to prepare 3,5-dimethoxybenzhydrol; (3) carrying out nucleophilic substitution on the 3,5-dimethoxy methyl benzoate and phosphorus tribromide, so as to generate 3,5-dimethoxy benzol benzyl bromide; (4) enabling the 3,5-dimethoxy benzol benzyl bromide to react through witting-horner, so as to prepare 3,5,4-triethoxy toluylene; and (5) preparing the trans-resveratrol from the 3,5,4-triethoxy toluylene. The total synthesis method has the advantages of being wide in material source, mild in reaction condition, friendly to environment, high in product yield, high in purity, applicable to industrialized mass production and the like.
Owner:HUNAN KEYUAN BIO PRODS
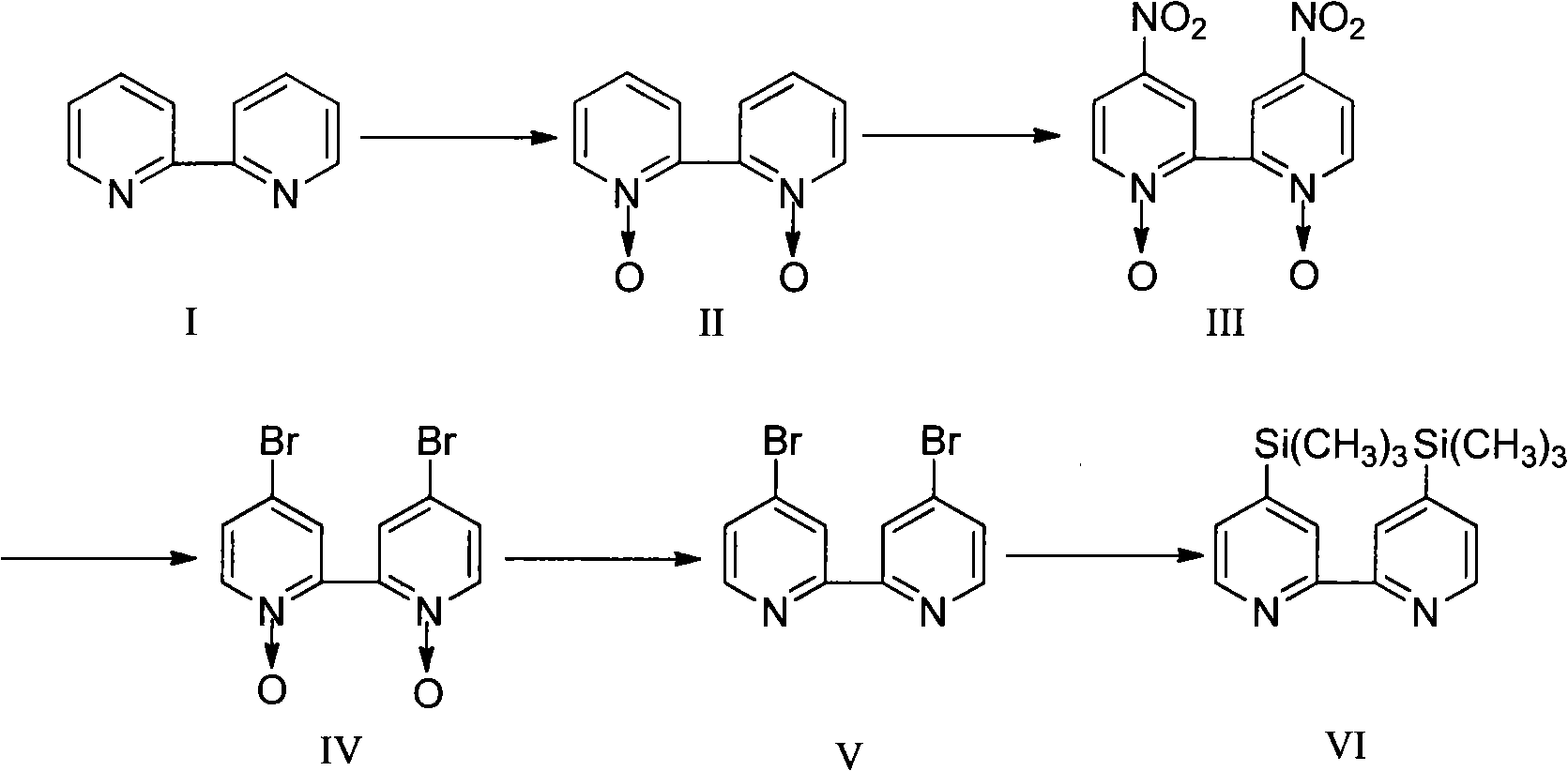
Synthesis method for 4, 4'-dibromo-2, 2'-dipyridyl
InactiveCN103130713AReduce dosageReduce adverse effectsOrganic chemistryPhosphorus tribromideSynthesis methods
The invention discloses a synthesis method for 4, 4'-dibromo-2, 2'-dipyridyl and belongs to the field of organic synthesis. The synthesis method includes the following steps: (A) oxidizing reaction: 2, 2'-dipyridyl reacts with 30% of hydrogen under the action of a metal catalyst and a phase transfer catalyst in the medium of water, and 2, 2'-dipyridyl nitrogen oxide is obtained; (B) nitration reaction: the 2, 2'-dipyridyl nitrogen oxide and fuming sulphuric acid are heated and react with fuming nitric acid in the medium of concentrated sulfuric acid, and 4, 4'-dinitro-2, 2'-dipyridyl nitrogen oxide is obtained; and (C) bromination reaction and deoxygenation reaction: the 4, 4'-dinitro-2, 2'-dipyridyl nitrogen oxide is added into acetic acid and heated till backflow, an acetyl bromide acetic acid solution is added, reaction is finished, a reducing agent is added, and the 4, 4'-dibromo-2, 2'-dipyridyl is obtained. According to the synthesis method for the 4, 4'-dibromo-2, 2'-dipyridyl, the solvent-free environment-friendly oxidizing reaction is adopted, the dosage of acetyl bromide is reduced, other reducing agents are used to replace phosphorus tribromide for the deoxygenation reaction, the bromination and reduction are changed into a one-pot method, harmful effects in the aspects of HSE (health, safety, environment) are reduced, and the synthesis method is environment-friendly and suitable for industrial production.
Owner:JIANGSU ZHONGDAN PHARMA RES +1
Method for synthesizing polysubstituted pyridin-2(1H)-one
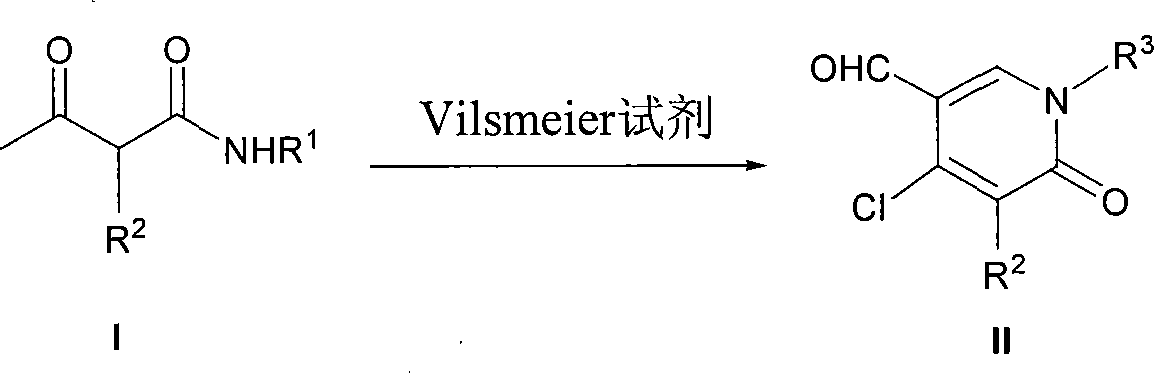
InactiveCN101121697AFew synthetic stepsRaw materials are easy to getOrganic chemistryPhosphorus tribromideSynthesis methods
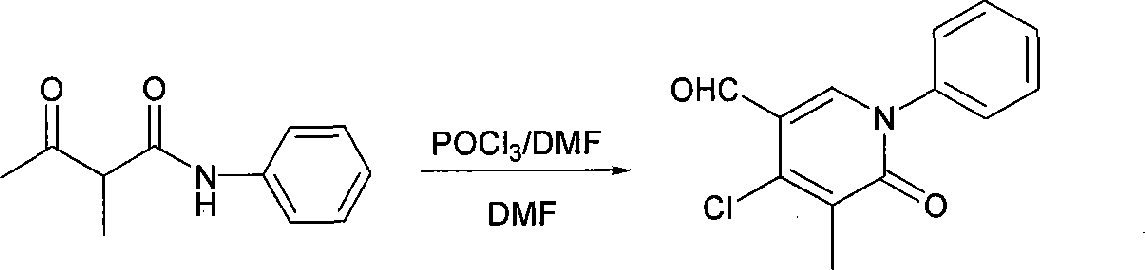
The invention belongs to an organic synthesis method, in particular relating to a synthesis method for the tic substitute pyridine-2 (1 H)-ketone compounds with the acetyl-acetylamine compounds under the reaction conditions of the Vilsmeier. The phosphorus oxychloride or phosphorus tribromide N, N-dimethylformamide mix and react to form the Vilsmeier reagent used in the reaction. The newly prepared Vilsmeier reagent is added into the reaction bottle provided with the return condenser and the blender; a reaction raw material (I) N, N-dimethylformamide solution expressed in the reaction formula is added into the system; the temperature is risen; the corresponding multi substituting pyridine-2 (1 H)-ketone compound (II) can be made after the column chromatography of the silica gel; the production rate is between 60 and 95 percent according to different reactions. The synthetic steps of the invention are fewer; the application scope is wide; the raw material is easy to get; the price is low; the functional group of the products is rich; the reaction conditions are mild; the production rate is high; the operation is easy; the invention is easy for the process of industrialization.
Owner:NORTHEAST NORMAL UNIVERSITY
High-yield synthesis method of sofosbuvir and sofosbuvir prepared with method
ActiveCN105646626AOvercome the conditionsOvercome by-productsSugar derivativesSugar derivatives preparationSodium methoxidePhosphorus tribromide
The invention relates to a high-yield synthesis method of sofosbuvir and sofosbuvir prepared with the method. The method comprises steps as follows: (a) cytidine and benzoic anhydride are dissolved in a first organic solvent for a reaction; TIDPSCl2 is added for a reaction; (b) a product and dimethyl phthalate are dissolved in a second organic solvent for a reaction; (c) a Grignard reagent is dissolved in tetrahydrofuran, and the temperature is reduced to subzero 35 DEG C to subzero 20 DEG C; tetrahydrofuran containing a third product is dropwise added and has a reaction, and a fourth product is obtained through purification; (d) the fourth product is dissolved in acetic acid containing tetrabutylammonium fluoride and has a reaction, and a sixth product is obtained through purification; (e) the sixth product is dissolved in the second solvent, phosphorus tribromide is added and has a reaction, and a seventh product is obtained through purification; (f) the seventh product is dissolved in methanol containing sodium methoxide and has a reaction, and an eighth product is obtained through purification. Accordingly, the reaction steps are reduced, the cost is greatly reduced, and the yield of the product is increased.
Owner:GUIZHOU INST OF TECH +1
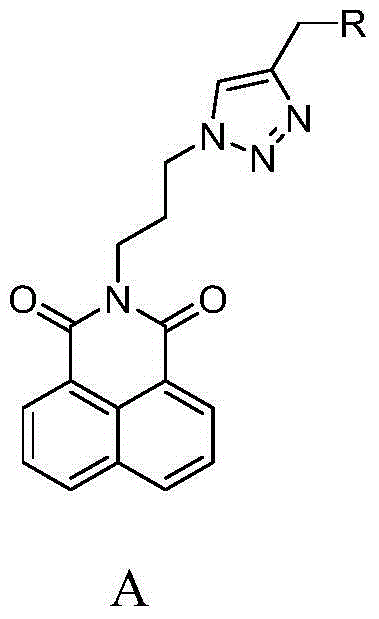
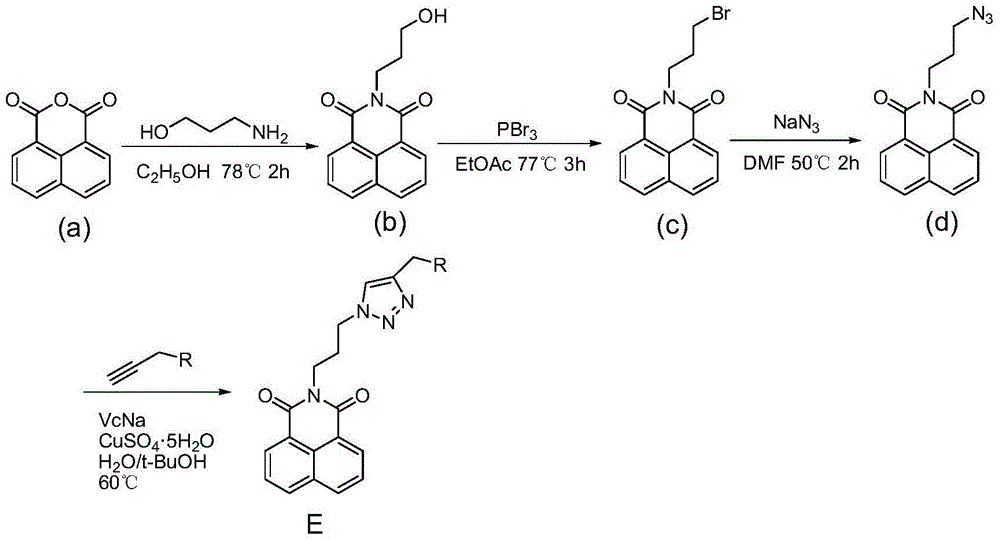
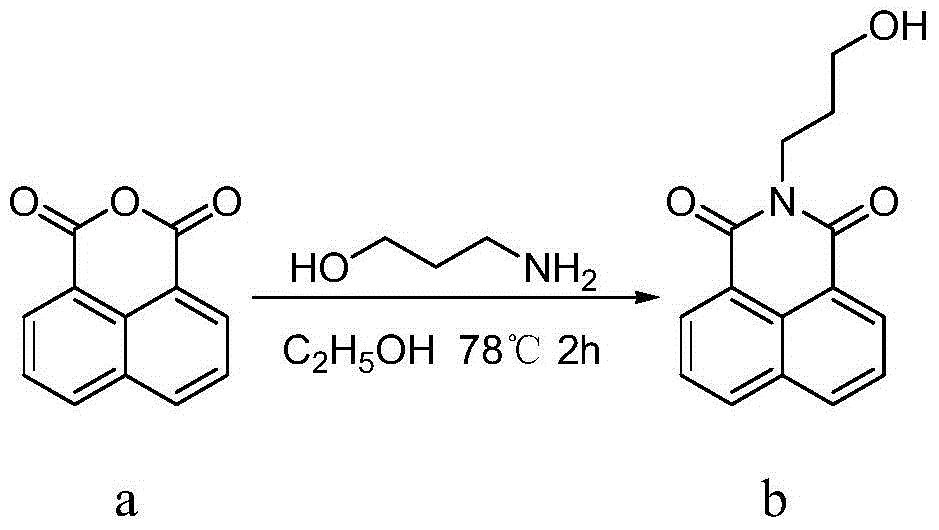
Synthesis and application of naphthalimide derivative containing 1,2,3-triazole on amide side chain
The invention relates to synthesis and an application of a naphthalimide derivative containing 1,2,3-triazole on an amide side chain, belonging to the field of organic synthesis. The derivative is a compound which has the structure shown in a general formula A. A preparation method of the derivative comprises the following steps: by taking anhydride naphthalene as a raw material, amidating the anhydride naphthalene with propyl alcohol amine, subsequently substituting hydroxyl by using phosphorus tribromide, then performing azidation of bromine and finally reacting with amino allylene connected with ring amine to prepare the naphthalimide derivative. The derivative is applied to a drug for inhibiting cancer cells.
Owner:DALIAN UNIV OF TECH
Preparation method and application of polymeric micelle with triple responsiveness
InactiveCN102766228AReduce volumeBreak the hydrophilic-hydrophobic balancePharmaceutical delivery mechanismPharmaceutical non-active ingredientsPolymer sciencePhosphorus tribromide
The invention relates to a preparation method and application of a polymeric micelle with triple responsiveness. The method comprises the following steps of: performing atom transfer radical polymerization on a polymerizable monomer dimethylaminoethyl methacrylate to obtain dimethylaminoethyl polymethacrylate, preparing pyrene methobromide from pyrene methanol and phosphorus tribromide, and grafting the pyrene methobromide to the dimethylaminoethyl polymethacrylate through quaterisation reaction to obtain the micelle with light, temperature and pH responsiveness. The polymeric micelle is stable at room temperature and can be loaded with hydrophobic molecules such as Nile red, the shape of the micelle is changed by ultraviolet irradiation and temperature and pH value regulation, and the loaded molecules are released from the micelle. The polymeric micelle has pH, temperature and light responsiveness and can have a wide application prospect in the field of medicine controlled release.
Owner:UNIV OF SCI & TECH BEIJING
Synthesis method of novel compound 4,4'-bis(trimethylsilyl)-2,2'-bipyridyl
InactiveCN102649797AEasy to operateThe materials are cheap and easy to getGroup 4/14 element organic compoundsPhosphorus tribromideOrganic synthesis
The invention belongs to the technical field of organic synthesis and particularly relates to a synthesis method of novel compound 4,4'-bis(trimethylsilyl)-2,2'-bipyridyl. According to the synthesis method, 2,2'-dipyridyl is utilized as an original raw material. The synthesis method comprises the following steps of: oxidation reaction: oxidating 2,2'-dipyridyl by utilizing an oxidizer, so as to generate a 2,2'-dipyridyl N,N'-oxide; nitration reaction: nitrating the 2,2'-dipyridyl N,N'-oxide in sulfuric acid by virtue of fuming nitric acid, so as to generate 4,4'-binitro-2,2'-dipyridyl N,N'-oxide; bromination reaction: performing bromine substitution reaction on the 4,4'-binitro-2,2'-dipyridyl N,N'-oxide in the presence of a brominating agent under an acid environment, so as to generate 4,4'-dibromo-2,2'-dipyridyl N,N'-oxide; deoxidation reaction: enabling the 4,4'-dibromo-2,2'-dipyridyl N,N'-oxide and phosphorus tribromide to react in a solvent, so as to generate 4,4'-dibromo-2,2'-dipyridyl; and trimethyl silicification reaction: enabling the 4,4'-dibromo-2,2'-dipyridyl and a trimethyl silanion solution to react, so as to obtain the 4,4'-bis(trimethylsilyl)-2,2'-bipyridyl. The synthesis method provided by invention has the advantages that the operation is simple, used materials are cheap and easily available, the yield is higher, the product purity is high, the product performance is excellent, and the method is better in development prospect and application potential.
Owner:JIANGNAN UNIV
Aloe-emodin quaternary ammonium salt alkyl iodoacetates with multiple-related anti-cancer mechanism
InactiveCN105924364AInhibit glycolysisDestroy energy supplyOrganic compound preparationQuinone preparationAntileukemic agentPhosphorus tribromide
The invention discloses aloe-emodin quaternary ammonium salt alkyl iodoacetates with the multiple-related anti-cancer mechanism and a preparation method thereof. The preparation method comprises the steps that firstly, aloe-emodin reacts with phosphorus tribromide to obtain brominated aloe-emodin; secondly, brominated aloe-emodin reacts with N-methyldi-n-octylamine to generate aloe-emodin quaternary ammonium salt; lastly, the aloe-emodin quaternary ammonium salt reacts with chloroacetyl chloride to generate iodine acetylation in the presence of sodium iodide to obtain aloe-emodin quaternary ammonium salt alkyl iodoacetates shown as a structural formula (please see the structural formula in the description). In-vitro cancer cell inhibition tests of aloe-emodin quaternary ammonium salt alkyl iodoacetates show that aloe-emodin quaternary ammonium salt alkyl iodoacetates has the good inhibitory activity on hematopoietic cells, is expected to be developed into anti-leukemia drugs and has the great application prospect.
Owner:FUZHOU UNIV
Extracting method for konjac glucomannan
The invention discloses the technical field of konjac production, and particularly relates to an extracting method for konjac glucomannan. The method comprises the steps that fresh konjac corm is cleaned, and roots, buds and peel are removed; the peeled konjac is cut into slices and placed into a phosphorus tribromide water solution to be soaked for 2-4 d; then, the soaked sliced konjac is ground into a sizing agent, water is added into the sizing agent and stirred till no flocculent precipitates are generated, then, standing is performed for 6 h or longer, bottom precipitates are removed, and a konjac glucomannan water solution is obtained; finally, ethyl alcohol is added into the konjac glucomannan water solution and stirred till no precipitates are generated, a konjac glucomannan ethyl alcohol solution is obtained, the konjac glucomannan ethyl alcohol solution is subjected to vacuum filtration to obtain konjac glucomannan precipitates, ethyl alcohol is used for washing the obtained high-purity konjac glucomannan. According to the extracting method, konjac glucomannan is adopted, water and ethyl alcohol are reasonably used, and the purposes of improving the extraction rate of the konjac glucomannan and keeping the original structure are achieved.
Owner:务川茂源现代农业开发有限公司
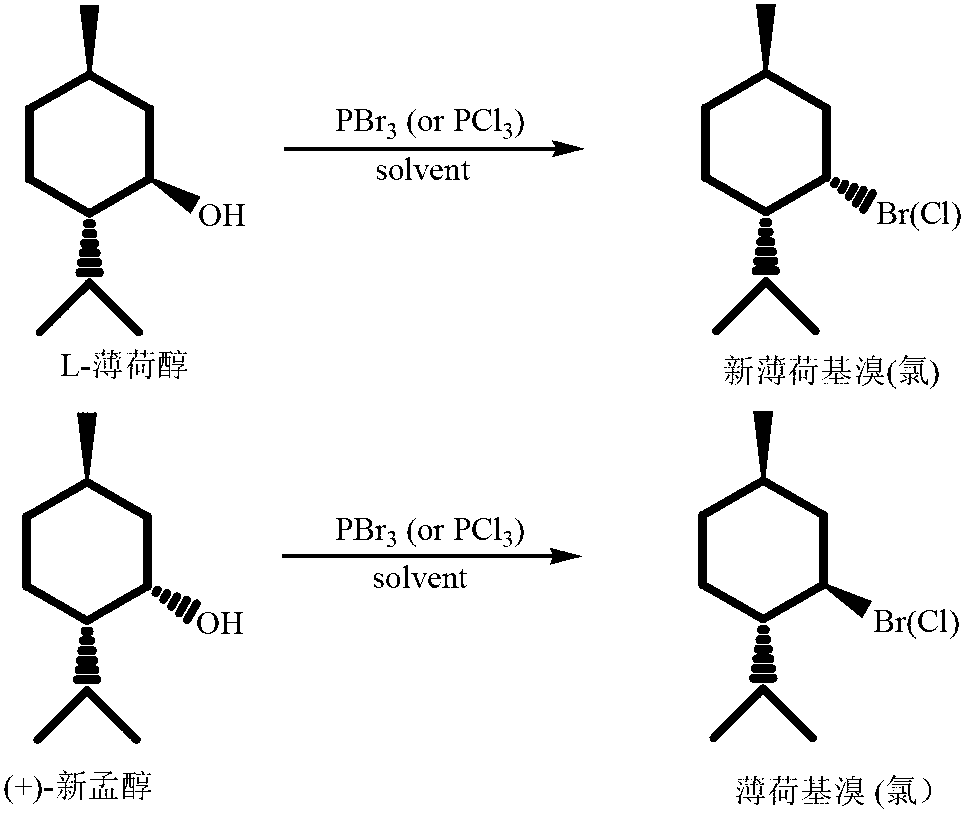
High-stereoselectivity method for synthesizing menthyl halide
ActiveCN103012049AEasy to prepareHigh selectivityHalogenated hydrocarbon preparationMentholPhosphorus tribromide
The invention discloses a high-stereoselectivity method for synthesizing menthyl halide, which comprises the following steps: adding menthol into a solvent in a nitrogen protective atmosphere, and dropwisely adding phosphorus tribromide or phosphorus trichloride in an ice bath, wherein the dropwise addition speed is controlled so that the reaction temperature is not higher than 5 DEG C; and after the dropwise addition, reacting at 0 DEG C for 2 hours, heating to 25 DEG C to react, detecting the reaction end point by TLC (thin layer chromatography), separating, washing, and drying to obtain the target product. The menthyl halide prepared by the method disclosed by the invention has the advantages of high yield and extremely high stereoselectivity, and the de value is greater than 99%.
Owner:ANHUI PROVINCE YIFAN SPICE
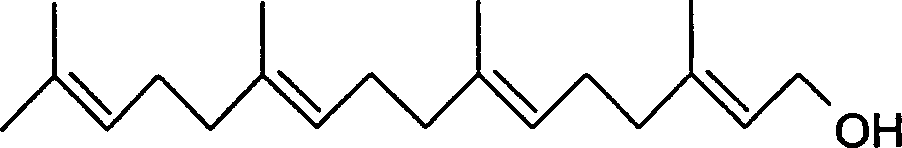
Synthesis of geranyl geraniol
InactiveCN1218918COrganic compound preparationHydroxy compound preparationPhosphorus tribromidePotassium
A process for synthesizing geranylgeraniol includes such steps as selectively oxidizing (E)-methyl of geranyl acetate by SeO2-tert-butyl hydrogen peroxide in dichloromethane to obtain mixed oxide, reducting by sodium borohydride in emthanol to obtain 8-geranyl hydroxy acetate, reacting on phosphorus tribromide in anhydrous ether under existance of pyridine to obtain trans-8-geranyl bromoacetate, condensation reacting on geranyl sulfone in N,N-dimethyl methylamide under existance of tert-butanol potassium to obtain 9-sulfogeranyl geraniol, and reducting by lithium in methylamine.
Owner:ZHEJIANG UNIV
Pentaerythritol-immobilized quinine catalyst as well as preparation method and application thereof
ActiveCN107552090AThe synthetic route is simpleHigh yieldOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsPentaerythritolPhosphorus tribromide
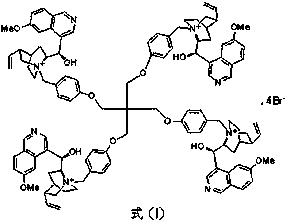
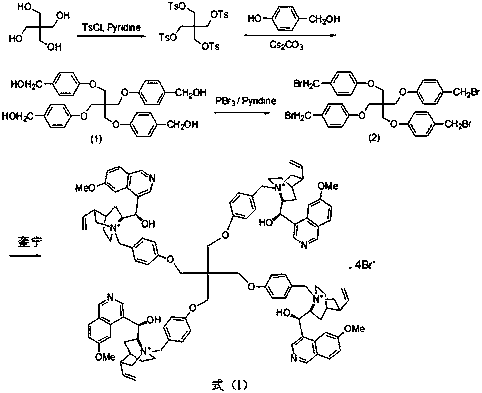
The invention discloses a pentaerythritol-immobilized quinine catalyst as well as a preparation method and application thereof. The structure of the pentaerythritol-immobilized quinine catalyst is asshown in a formula (I). The preparation method of the pentaerythritol-immobilized quinine catalyst comprises the following steps of a, enabling pentaerythritol to react with para-toluene sulfochlorideto prepare pentaerythritol besilate; afterwards, reacting with p-hydroxybenzyl alcohol in an alkaline condition to prepare an intermediate (1); b, under the action of pyridine, enabling the intermediate (1) to react with phosphorus tribromide, so as to prepare an intermediate (2); c, enabling the intermediate (2) to react with quinine, so as to prepare the pentaerythritol-immobilized quinine catalyst. The pentaerythritol-immobilized quinine catalyst can be applied to the asymmetric epoxidation reaction of chalcone, and the catalyst is separated and purified simply, and can be repeatedly recycled. The formula (I) is shown in the description.
Owner:东营睿港招商服务有限责任公司
Technique for preparing hexabromocyclophosphazene trimer
InactiveCN101265276AIncrease success rateMild reaction conditionsGroup 5/15 element organic compoundsChlorobenzenePhosphorus tribromide
The invention discloses a novel process for preparing hexabromocyclotriphosphazene. The process is characterized in charging step by step and including allowing ammonium bromide, phosphorus tribromide and liquid bromine to react in chlorobenzene in the presence of high efficiency polybasic composite catalyst, and purifying the product through freeze separation. The product has a purity of greater than 98% and a yield of about 50%. Compared with the conventional method, the process has the advantages of short reaction time and high product yield.
Owner:HEBEI UNIVERSITY
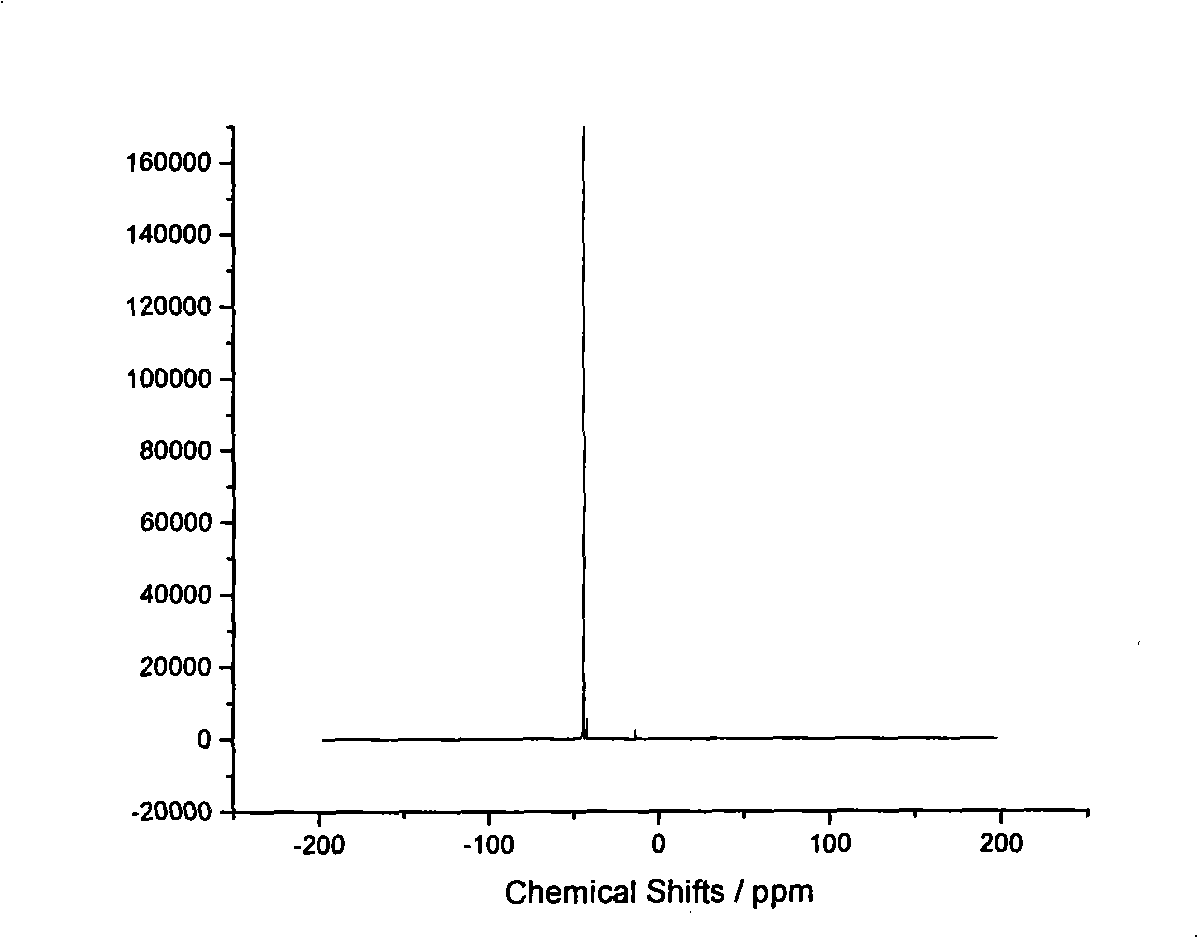
Preparation method of trialkyl phosphine borofluoride
InactiveCN103012477AHigh yieldSimple methodGroup 5/15 element organic compoundsPhosphorus tribromideGrignard reagent
The invention discloses a preparation method of trialkyl phosphine borofluoride. The preparation method comprises the following steps of: reacting a raw material, alkyl Grignard reagent, with phosphorus tribromide at a lower temperature; adding fluoboric acid into the obtained object for salification; and carrying out extraction, concentration and recrystallization on the obtained product, and cooling the obtained product so as to separate out the trialkyl phosphine borofluoride. The preparation method disclosed by the invention is simple and easy to implement, is safe and environmental-friendly, and is low in cost; the yield of the trialkyl phosphine borofluoride prepared by the method with the alkyl Grignard reagent and phosphorus tribromide is high and can be up to 49%-55%, and an organic solvent extracted in the reaction can be directly recycled. The method disclosed by the invention also has the innovation that trialkyl phosphine compounds sensitive to air are converted into borofluoride with stable properties in air, and the trialkyl phosphine borofluoride is convenient to use and store and has a wide application range.
Owner:DALIAN NETCHEM CHIRAL TECH
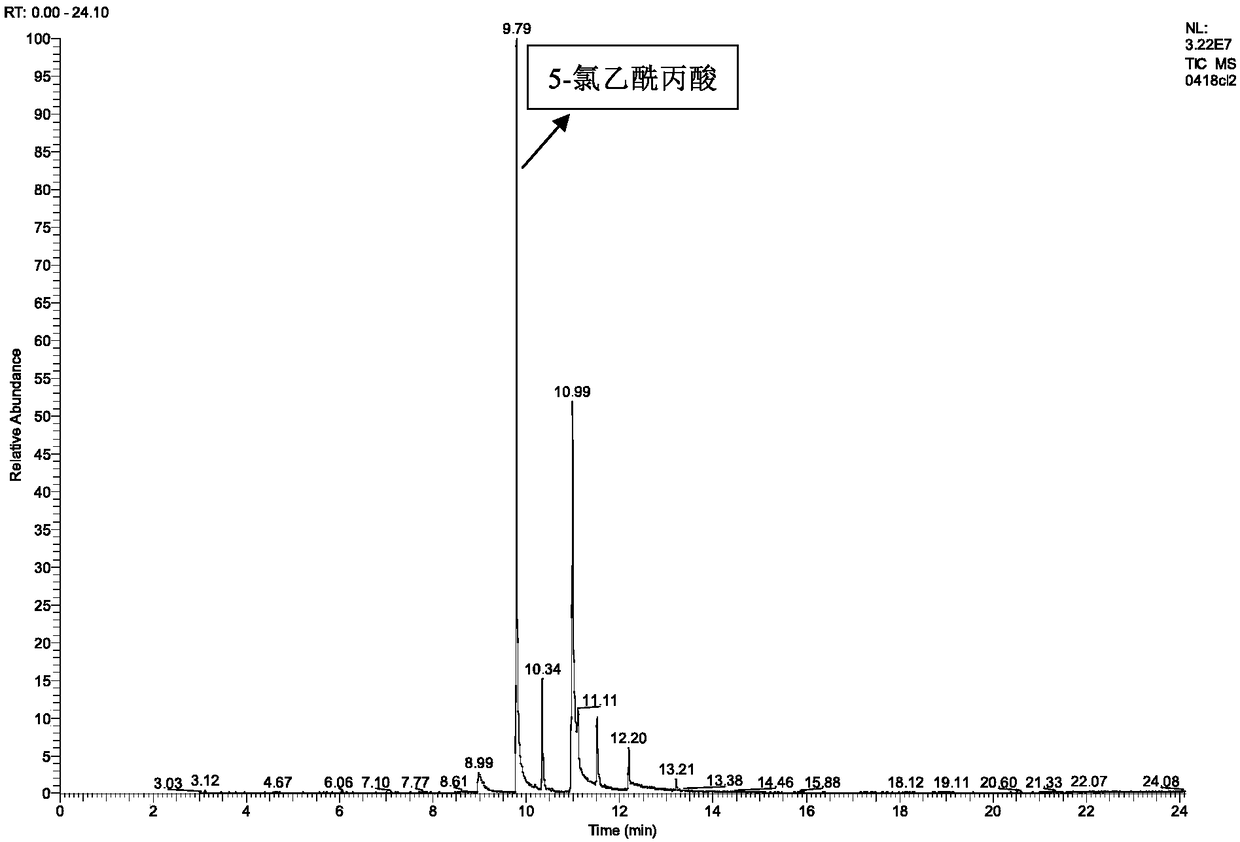
Preparation method of 5-haloacetyl propionate
InactiveCN108358789AWide variety of sourcesHigh reaction yieldOrganic compound preparationCarboxylic acid esters preparationPhosphorus tribromideN-Chlorosuccinimide
The invention discloses a preparation method of 5-haloacetyl propionate. Acetylpropionic acid or acetyl propionate and a halogenating agent are taken as raw materials, and the halogenating agent includes NBS (N-Bromosuccinimide), NCS (N-Chlorosuccinimide), ferrous bromide, iron bromide, aluminum bromide, phosphorus tribromide, copper bromide and copper chloride. The acetylpropionic acid or acetylpropionate obtained by hydrolysis of biomass is taken as a raw material, and undergoes a halogenation reaction in the presence of a halogenating agent to synthesize the 5-haloacetyl propionate. The selected raw materials are wide in sources, cheap and readily available; the reaction conditions are mild, and the reaction yield and selectivity are high; moreover, the selected halogenating agent serving as a raw material is less toxic and more environmentally friendly than liquid bromine used in the traditional mercury process, and has a good industrial application prospect.
Owner:XIAMEN UNIV
Edible flavor difurfuryl thioether
InactiveCN101885714ARaw materials are easy to getSimple and fast operationOrganic chemistryFood preparationPhosphorus tribromideSolvent
The invention relates to edible flavor difurfuryl thioether. The edible flavor difurfuryl thioether comprises the following raw materials in part by weight: 40 to 45 parts of furfuryl alcohol, 40 to 45 parts of phosphorus tribromide, 43 to 45 parts of furfurylmercaptan and 75 parts of sodium hydroxide, wherein 560ml of absolute ether and 800ml of ether are used as a solvent and an extraction agent respectively. Because the flavor has burnt baking smell, the flavor serves as an important flavoring material in meat flavors, can be widely used as flavorings for instant noodles and various meat products and can also be used for baked foods, icy foods, milk foods, puddings and the like; and due to the pure, vivid, thick and mellow smell, rich meat feel, good mouthfeel, the flavor is widely used, is a novel meaty flavor and is also an ideal choice for cans, sauces, convenience foods and condiments.
Owner:TIANJIN CHEM REAGENT RES INST
A kind of synthetic method of bromiesoval
InactiveCN102276504AHigh yieldHigh purityUrea derivatives preparationOrganic compound preparationHydrobromidePhosphorus tribromide
The invention discloses a method for synthesizing bromisoval. The first step: Isovaleric acid reacts with bromine under the catalysis of phosphorus tribromide to generate α-bromoisovaleric acid; the second step: after the first step, the product is directly pressed into the acid bromide without separation. In the tank, add phosphorus tribromide and bromine, and continue the reaction to generate α-bromoisovaleryl bromide. After the reaction, collect the 85-95℃ / 20-30mmHg fraction by distillation under reduced pressure and temperature to obtain α-bromoisovaleryl bromide Valeryl bromide; the third step: condensation of α-bromoisovaleryl bromide and urea to obtain bromisoval. The invention has the advantages of high product yield, high purity, less impurities, low cost, easy production control, hydrogen bromide gas generated in the production process is absorbed by water to obtain hydrobromic acid, which can be comprehensively utilized, and the discharge of three wastes is reduced.
Owner:SHANDONG FANGMING PHARMACEUTICAL CO LTD
Pitavastatin calcium intermediate preparation method
PendingCN110407818AAvoid it happening againImprove protectionOrganic chemistryPhosphorus tribromideQuinoline
The invention discloses a pitavastatin calcium intermediate preparation method, and relates to the technical field of preparation of pitavastatin calcium intermediates. In the prior art, the Wittig reaction can generate a large amount of solid waste triphenyl phosphorus oxychloride, and the solid waste is difficult to completely remove through post-treatment purification. A purpose of the presentinvention is to solve the problem in the prior art. The preparation method comprises: 1, carrying out a reaction on (2-cyclopropyl-4-(fluorophenyl)quinoline-3-yl)methanol I and phosphorus tribromide in dichloromethane to form 3-(bromomethyl)-2-cyclopropyl-4-(4-fluorophenyl)-3-quinoline II, and extracting with dichloromethane; 2, carrying out a Reformatsky reaction on the 3-(bromomethyl)-2-cyclopropyl-4-(4-fluorophenyl)-3-quinoline II and 2-((4R,6S)-6-formyl-2,2-dimethyl-1,3-dioxane-4-yl)tert-butyl acetate to obtain an alcohol VII; and 3, adding p-toluenesulfonyl chloride in a dropwise manner to obtain p-toluenesulfonate VIII, treating the reaction liquid with potassium tert-butoxide, and carrying out an elimination reaction to obtain 2-((4R,6S)-6-((E)-2-(2-cyclopropyl-4-(4-fluorophenyl)quinoline-3-yl)vinyl)-2,2-dimethyl-1,3-dioxane-4-yl) tert-butyl acetate V, or directly treating with a sodium carbonate aqueous solution to obtain the product V.
Owner:安庆恩聚生物医药科技有限公司
Chemical synthesis process for preparing gastrodin and similar phenolic glucoside thereof
InactiveCN105646610AIncrease conversion rate per passHigh yieldSugar derivativesSugar derivatives preparationChemical synthesisAcetic anhydride
The present invention discloses a chemical synthesis process for preparing gastrodin and similar phenolic glucoside represented by a formula (I) thereof. According to the present invention, industrial easily-available raw materials such as acetic anhydride and acetyl bromide or PHOSPHORUS TRIBROMIde or phosphorus pentabromide are used to complete the hydroxy protection of glycosyl and the bromination reaction of hemiacetal hydroxy, such that the problems that the use of red phosphorus and bromine during the gastrodin bulk drug production process causes physical and psychological harm on production workers and environment pollution and damage are solved; and the process mainly comprises two steps of key reactions, wherein the first step reaction is synthesis of bromoacetyl glycosyl compound, and the second step reaction is condensation of the bromoacetyl glycosyl compound and a phenolic compound to generate the acetyl-protected phenolic glucoside compound.
Owner:赵建英
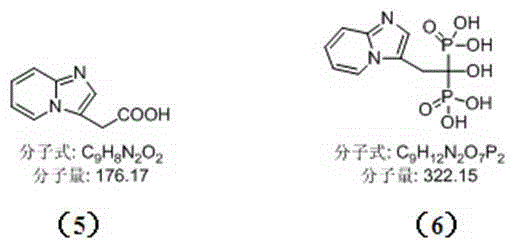
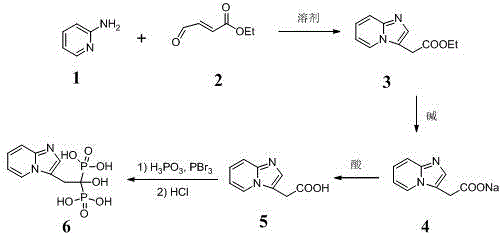
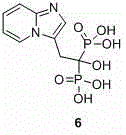
Method for synthetizing minodronic acid intermediate and preparing minodronic acid by virtue of one-pot process
InactiveCN104693241AEasy to operateMild reaction conditionsGroup 5/15 element organic compoundsPhosphorous acidPhosphorus tribromide
The invention relates to a preparation technology of a minodronic acid key intermediate 2-{imidazo[1,2-a]pyridine-3-yl}acetic acid (compound 5), and a monohydrate prepared by synthesizing the minodronic acid (6) by employing the intermediate, purifying and refining. The synthesis of the minodronic acid key intermediate has an important effect on the quality of the minodronic acid. The technology comprises the following steps: firstly, with 2-aminopyridine (1) and trans-4-amino-2-ethyl crotonate (2) as raw materials, synthesizing a high-purity minodronic acid intermediate (5) through the treatment such as hydrolysis and acidification, wherein the reactions are finished by one-pot reaction; and then synthesizing the minodronic acid (6) under the action of phosphorous acid and phosphorus tribromide.
Owner:BEIJING VENTUREPHARM BIOTECH
Synthetic method of triolefin insect sex attractant
InactiveCN103262841AReduce pollutionShort processBiocidePest attractantsPhosphorus tribromideGrignard reagent
The invention discloses a synthetic method of a triolefin insect sex attractant. The synthetic method comprises the steps of (1) enabling alpha-ethyl linolenate to react with absolute ether to generate alpha-linoleny alcohol under catalytic action of LiAlH4; (2) enabling the alpha-linoleny alcohol to react with phosphorus tribromide to generate cis, cis, cis-1-bromine-9,12,15-gadusene in the presence of an organic solvent with weak polarity; and (3) enabling the cis, cis, cis-1-bromine-9,12,15-gadusene to react with a grignard reagent to generate cis, cis, cis-3,6,9-Cn triene under the effects of tetrahydrofuran, lithium tetrachlorocuprate and N-methyl pyrrolidone. By adopting the synthetic method of the triolefin insect sex attractant disclosed by the invention, an insect in-vivo biosynthetic pathway is simulated; polyene hydrocarbon information sex pheromone components of C19, C20 and C21 are synthesized by taking cheap and available alpha-ethyl linolenate as the material; and the synthetic method is short in technologic process, mild in synthetic reaction condition, short in reaction time, high in yield, simple and convenient to operate, wide in application range, low in cost, fewer in three wastes, little in environmental pollution, and easy to industrialize, and the product can be easily separated.
Owner:SHANXI AGRI UNIV
Method for synthesizing bromotrimethylsilane
ActiveCN102153582AAvoid it happening againHigh purityGroup 4/14 element organic compoundsPhosphorus tribromideChemical products
The invention discloses a method for synthesizing bromotrimethylsilane, and relates to a synthetic production technology for a chemical product. The method comprises the following steps of: under oxygen-free conditions, stirring in a reaction kettle in which phosphorus tribromide and silyl ether are sequentially added, raising the temperature to 1005 DEG C, keeping the temperature, performing reflux reaction for 60.5 hours, rectifying under normal pressure to obtain the bromotrimethylsilane, and stopping to receive a finished product of the bromotrimethylsilane when the temperature in the kettle is raised to 135 DEG C. By the method, a silyl ether-phosphorus tribromide method is improved and innovated, side reactions are avoided, the obtained product has purity, and the finished product of the bromotrimethylsilane which has the content of about 99 percent can be obtained; and the stirring at normal temperature is changed into high-temperature reflux, so that the reaction time is greatly shortened.
Owner:扬州三友合成化工有限公司
Spiroindane skeleton chiral quaternary ammonium salt as well as preparation method and application thereof
PendingCN114262295ARich varietyOrganic-compounds/hydrides/coordination-complexes catalystsImino compound preparationGlycinePhosphorus tribromide
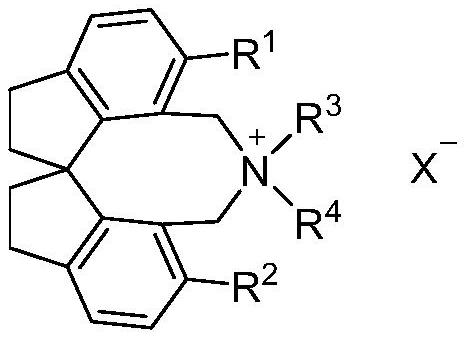
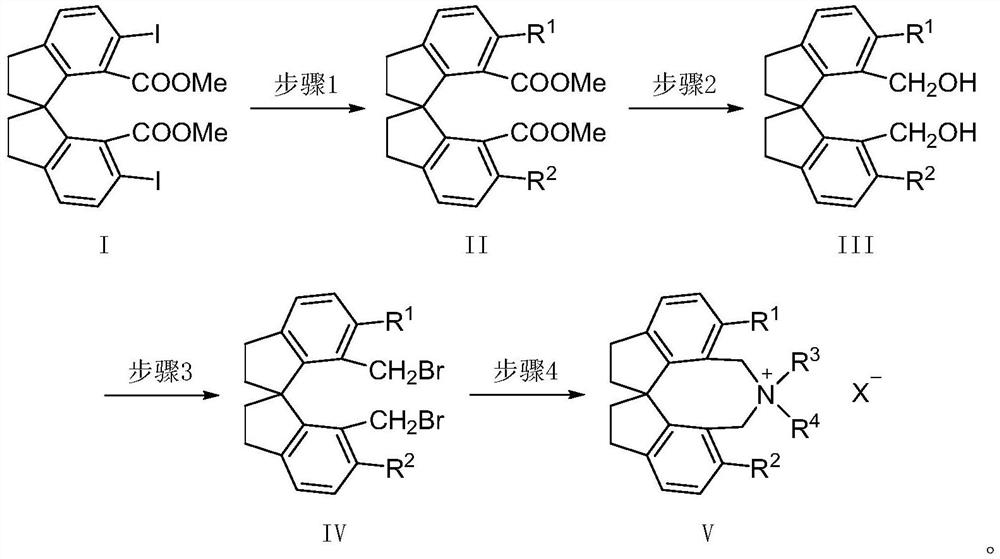
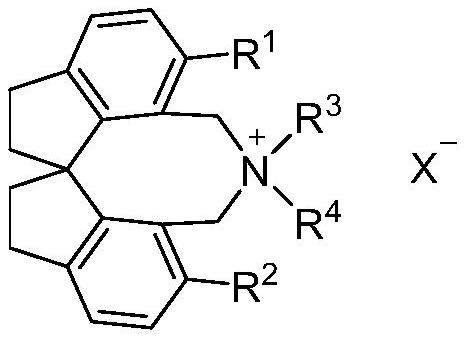
The invention relates to spirobiindane skeleton chiral quaternary ammonium salt as well as a preparation method and application thereof. The preparation method comprises the following steps: under the action of bis (triphenylphosphine) palladium chloride and potassium carbonate, enabling optically active 6, 6 '-diiodo-1, 1'-spirobiindane-7, 7 '-dimethyl diformate to react with arylboronic acid to generate 6, 6'-disubstituted-1, 1 '-spirobiindane-7, 7'-dimethyl diformate; the preparation method comprises the following steps: reacting 6, 6 '-disubstituted-1, 1'-spirobiindane-7, 7 '-dimethanol with diisobutylaluminium hydride to generate 6, 6'-disubstituted-1, 1 '- The preparation method comprises the following steps: reacting 7, 7 '-disubstituted-7, 7'-bis (bromomethyl)-1, 1 '-spirobiindene with phosphorus tribromide to generate 6, 6'-disubstituted-7, 7 '-bis (bromomethyl)- And reacting with secondary amine under the action of potassium carbonate to generate corresponding chiral quaternary ammonium salt. The spirobiindane skeleton chiral quaternary ammonium salt can be used for catalyzing an asymmetric alkylation reaction of diphenylmethylene glycine tert-butyl ester and pentafluorobenzyl bromide, the highest yield of the obtained product can reach 98%, and the highest enantioselectivity can reach 95% ee.
Owner:TIANJIN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com