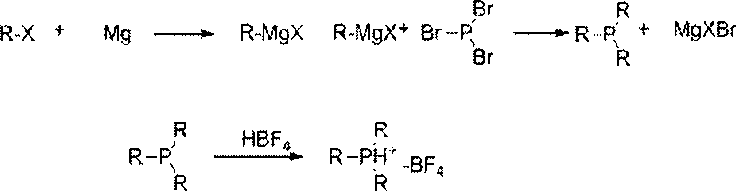Preparation method of trialkyl phosphine borofluoride
A technology of alkylphosphine fluoroborate and fluoroboric acid, which is applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, organic chemistry, etc., and can solve bad smell, unfriendly environment, purchase restrictions, etc. problems, to achieve the effect of convenient use and storage, simple method and wide application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0018] A method for preparing trialkylphosphine fluoroborate, the raw material alkyl Grignard reagent reacts with phosphorus tribromide at low temperature, after adding fluoroboric acid to form a salt, extraction, concentration, recrystallization, and cooling to precipitate trialkylphosphine fluoroboron salt.
[0019] Reaction mechanism of the present invention is as follows:
[0020]
[0021] In the formula, R: alkyl or aromatic hydrocarbon group.
[0022] Specific process:
[0023] Add 76.8g magnesium and 300g cyclohexyl chloride tetrahydrofuran solution to a 2000 mL round bottom flask equipped with a nitrogen protection device and mechanical stirring. Control the temperature ≤ 40°C, add iodine to trigger, add cyclohexyl chloride tetrahydrofuran solution dropwise with a constant pressure dropping funnel, control the temperature at 60-65°C, after the addition is complete, stir and reflux for 2 hours. Under the protection of argon, configure 110.2g of phosphorus tribro...
Embodiment 2
[0025] A method for preparing trialkylphosphine fluoroborate, which is the same as the method and reaction mechanism of Example 1, and the specific process:
[0026] Under the protection of nitrogen, add 7.28g (1.05eq) magnesium chips and 15.2g (1 / 8 of the total weight) chlorobutane THF solution (26.7g chlorocyclohexane dissolved in 135mL THF) into a 250mL four-necked bottle , heat up to 55-60°C, add 2 grains of iodine to trigger successfully, add dropwise the remaining 105.5g (total 1.0eq) chlorobutane tetrahydrofuran solution, while maintaining boiling reflux, after the dropwise addition, heat to reflux for 2h, Cool down to room temperature for later use, and analyze and measure the concentration to be 1.82±0.3mmol / g (about 154g). In a 500mL four-neck flask, under the protection of argon, put 7.5g (0.182eq) of phosphorus tribromide and 70mLTHF, and stir at room temperature. 988mg (0.0182eq) of cuprous iodide and 898mg (0.0364eq) of lithium bromide, lower the temperature to -...
Embodiment 3
[0029] A method for preparing trialkylphosphine fluoroborate, which is the same as the method and reaction mechanism of Example 1, and the specific process:
[0030] Under the protection of nitrogen, add 24.3g (1.05eq) magnesium chips and 60.8g (one-eighth of the total weight) chlorobutane THF solution (88g chlorocyclohexane dissolved in 450mL THF) into a 1000mL four-necked bottle, Heat up to 55-60°C, add 2 grains of iodine to trigger successfully, add dropwise the remaining tert-chlorobutane solution while maintaining boiling reflux, after the dropwise addition, heat to reflux for 2 hours, cool down to room temperature for later use, under nitrogen protection, Prepare 47.8g of phosphorus tribromide and 226mLTHF (water content 0.01%), cool down to -20°C with dry ice ethanol, add the Grignard reagent dropwise to phosphorus trichloride, control the temperature at -15°C to -10°C, and complete the dropwise addition After that, raise the temperature to room temperature, stir for 3 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com