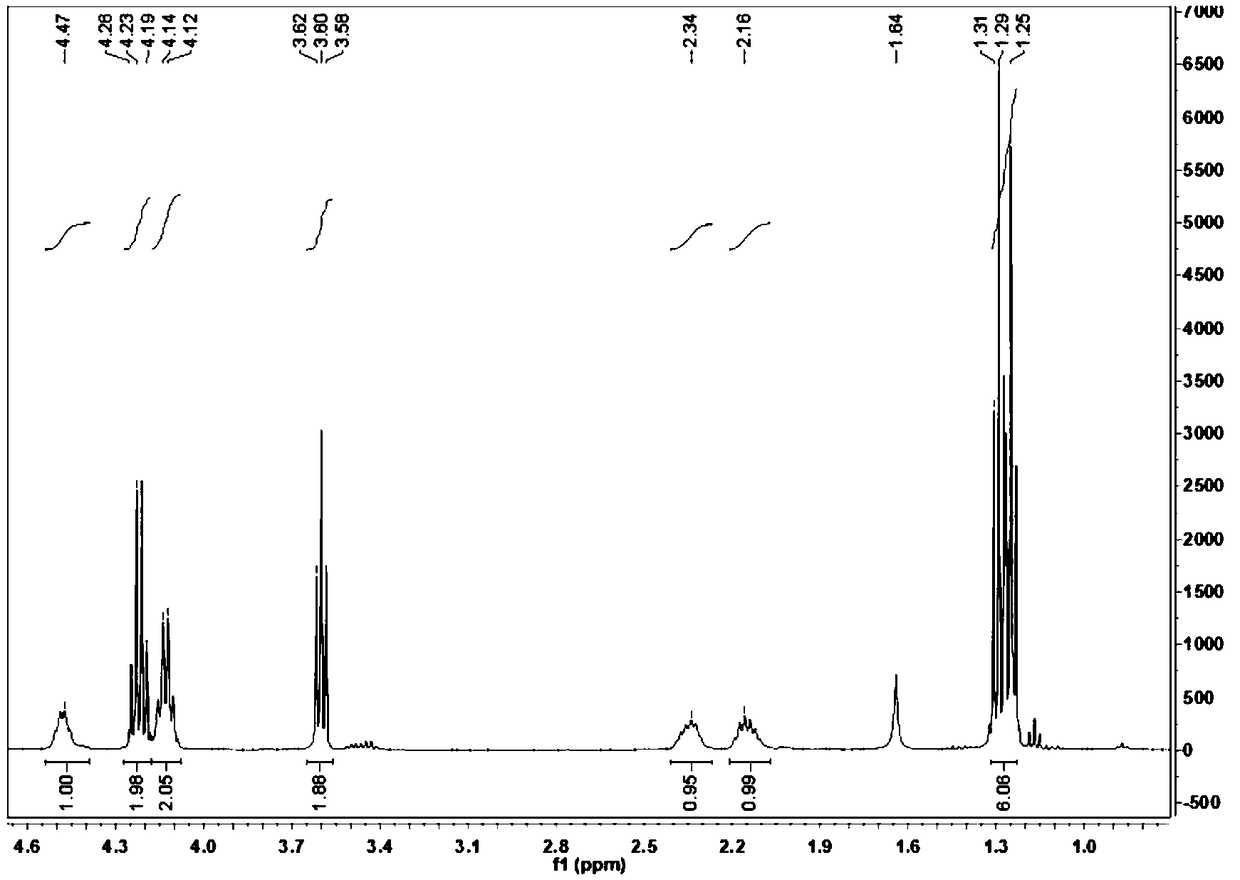
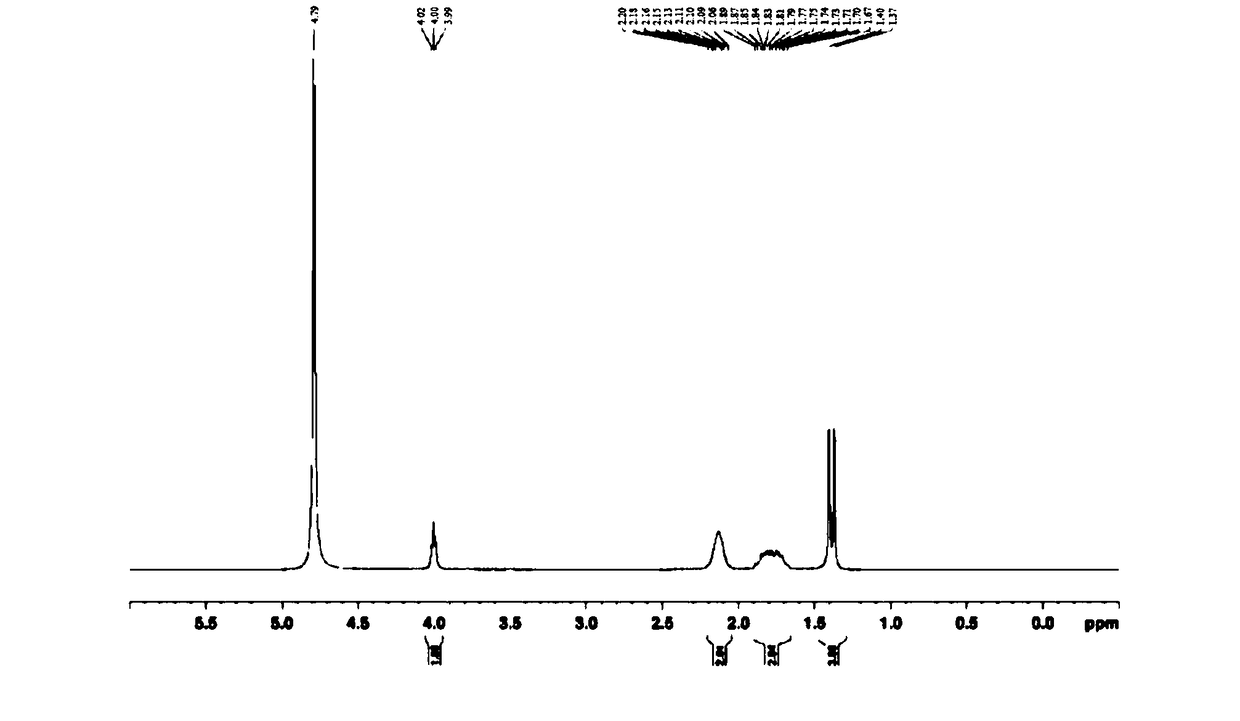
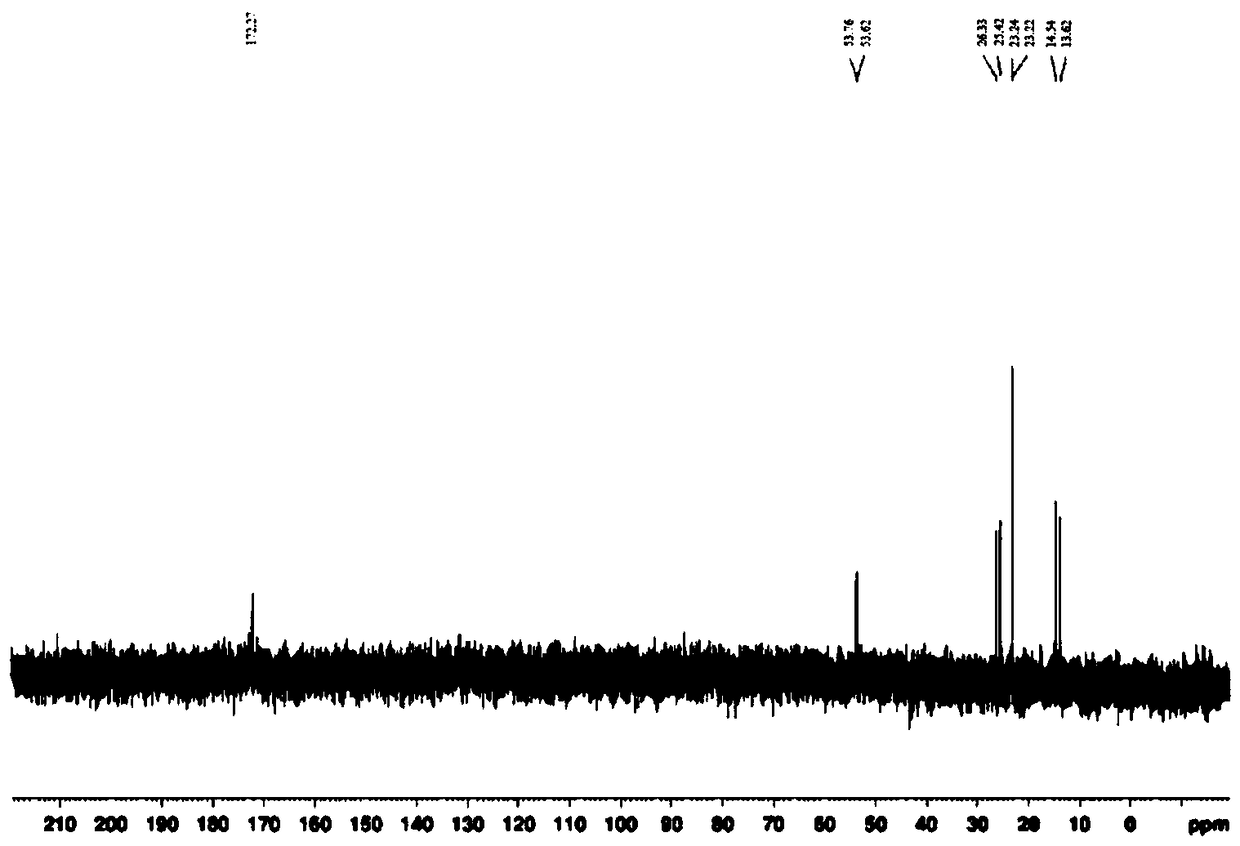
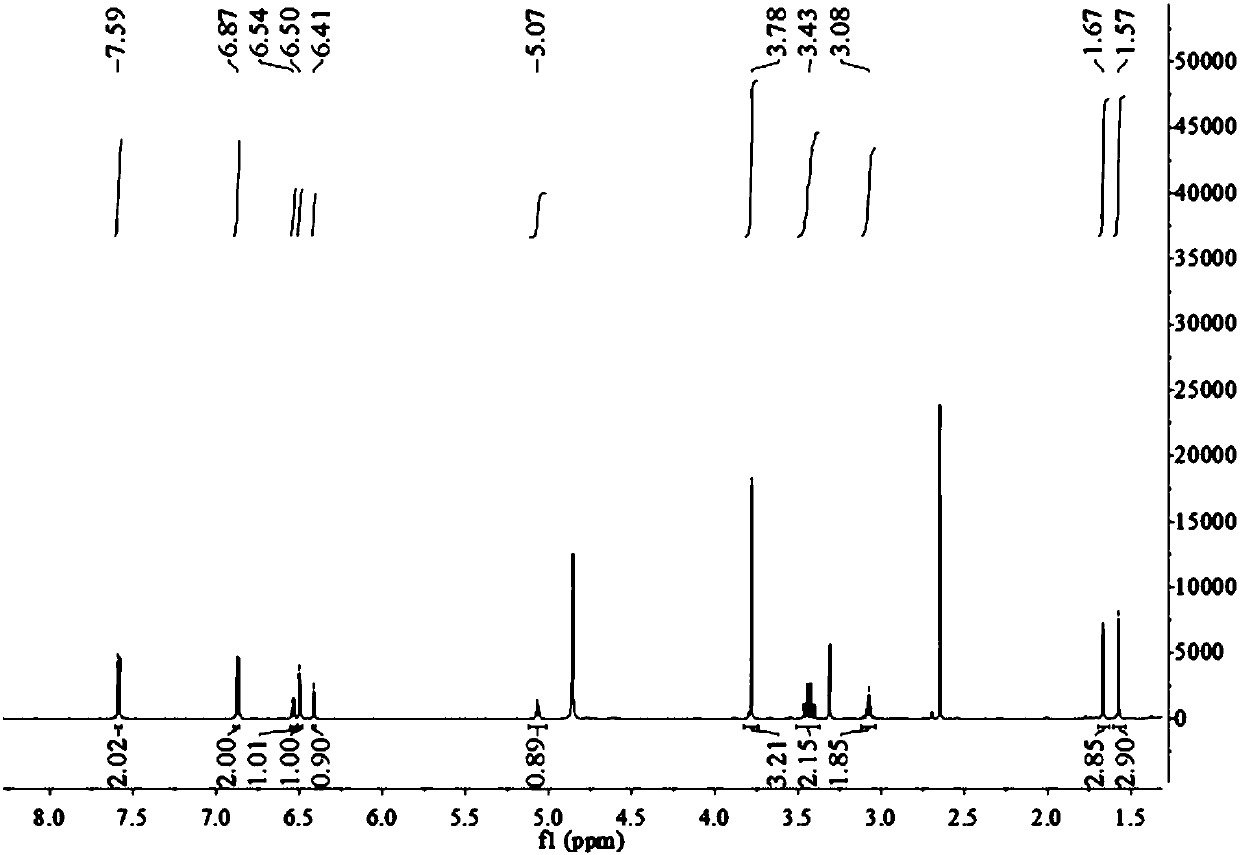
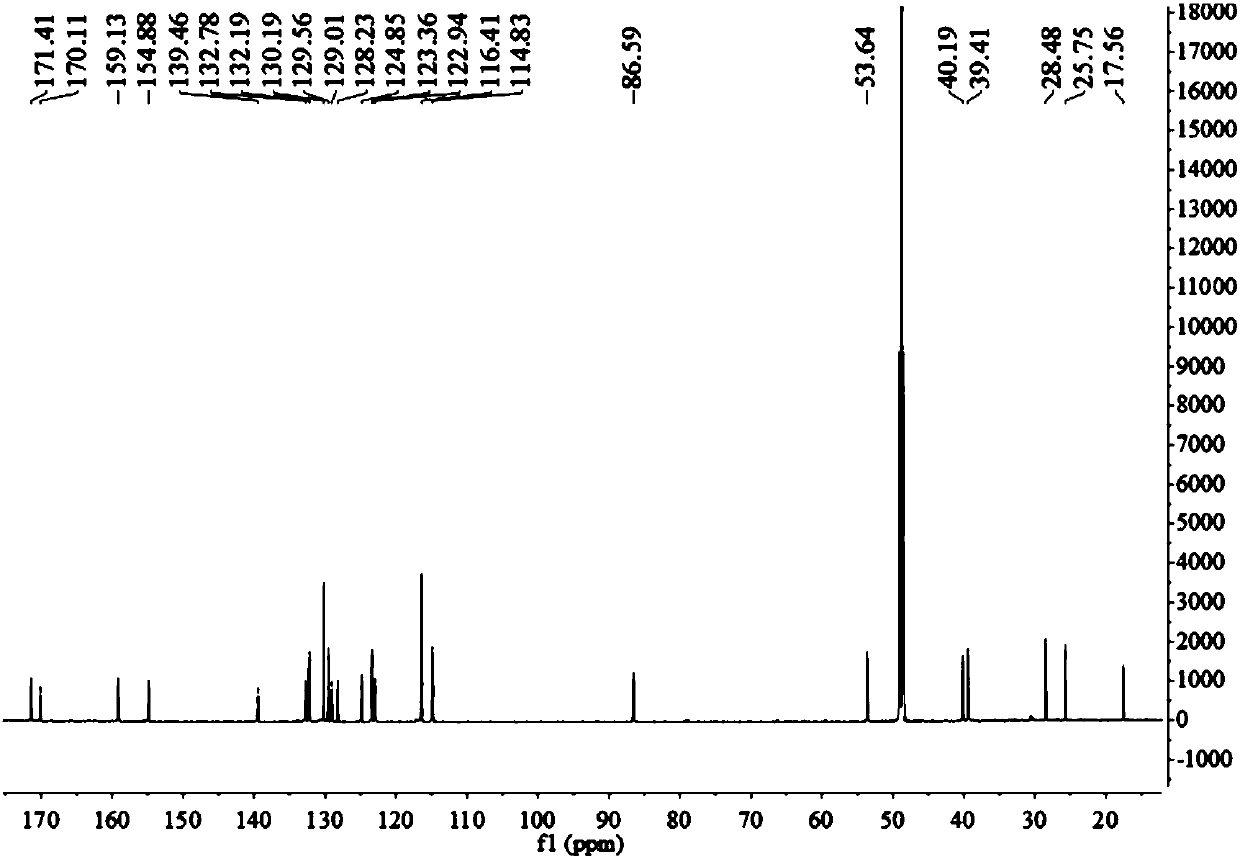
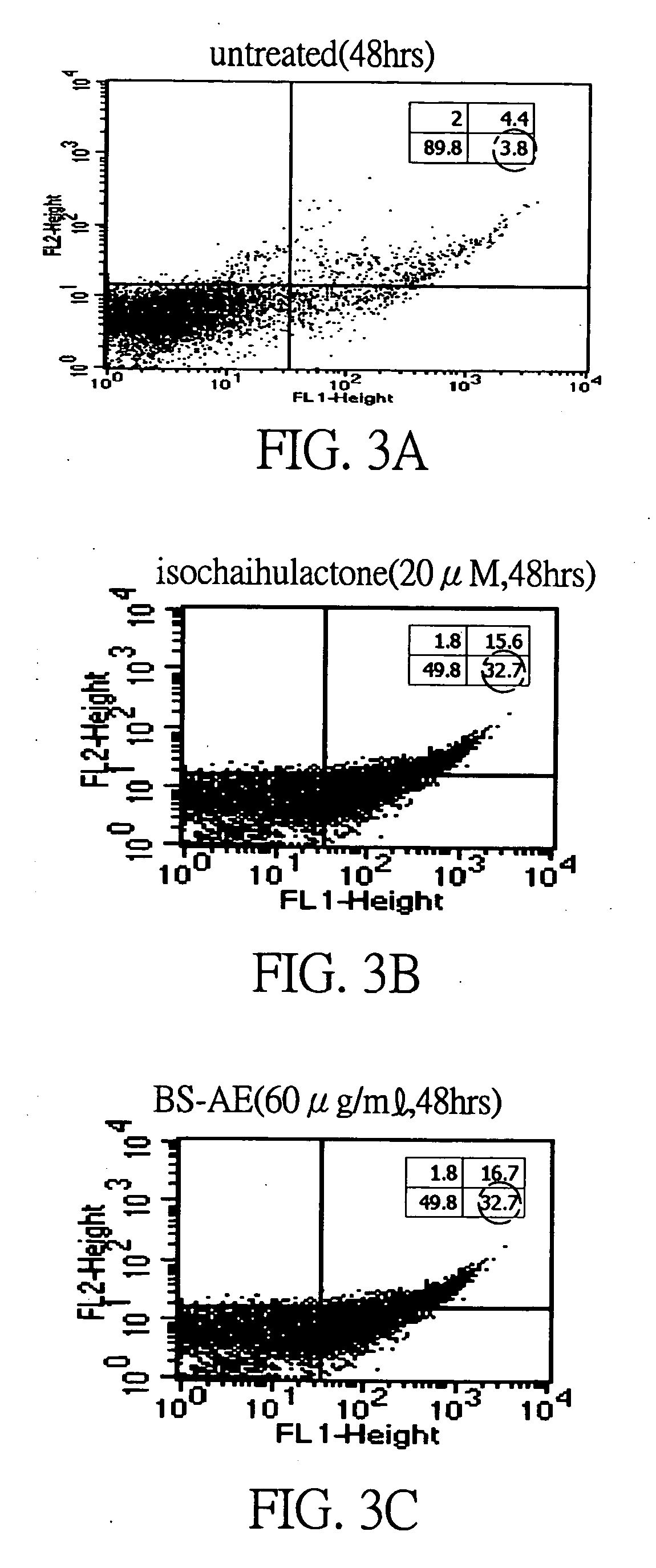
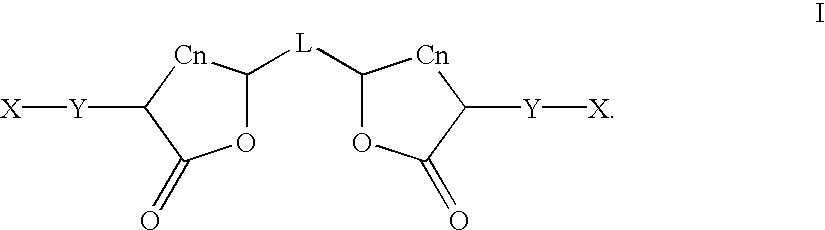
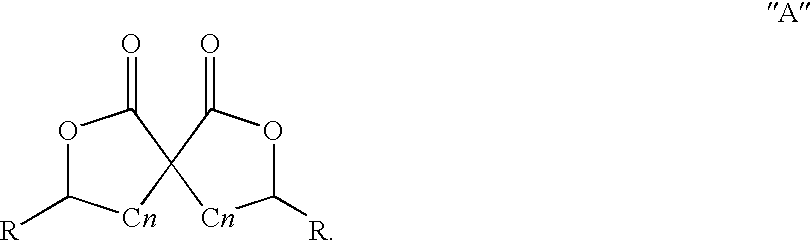
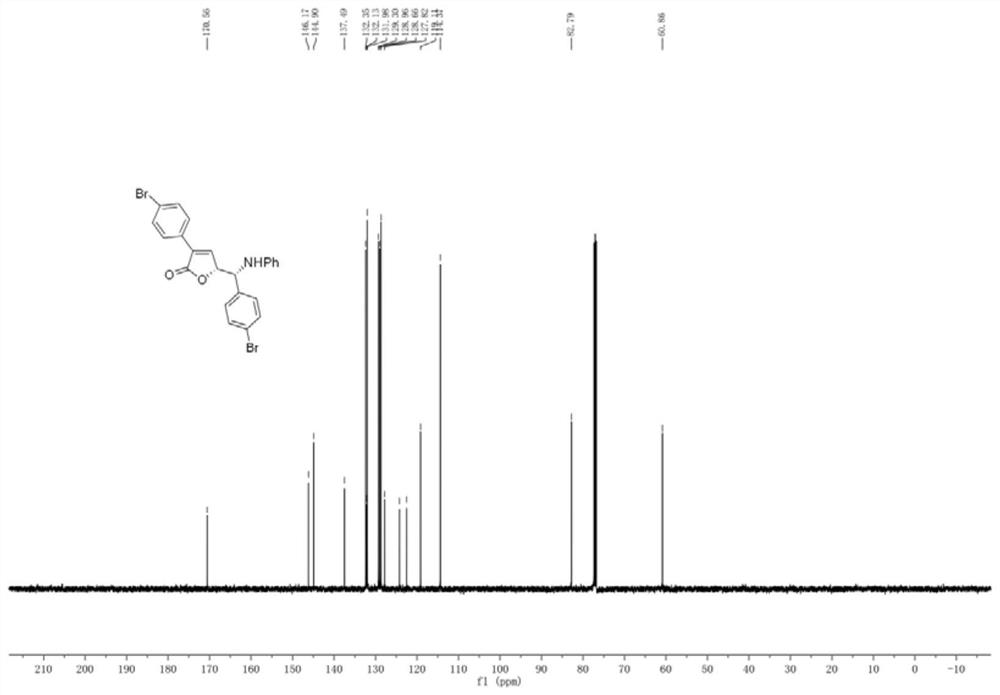
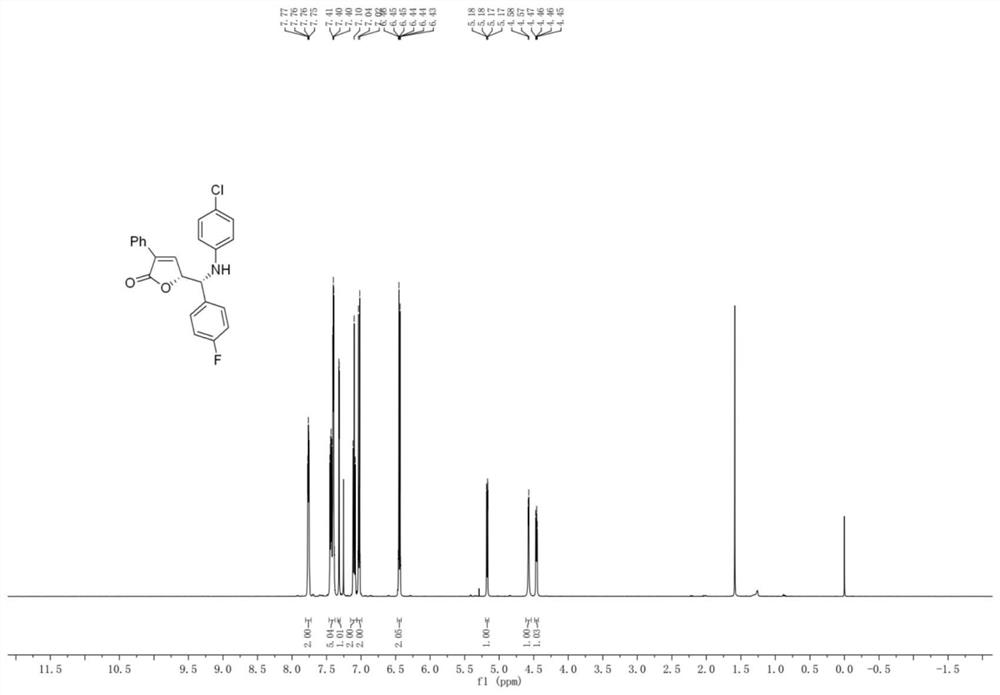
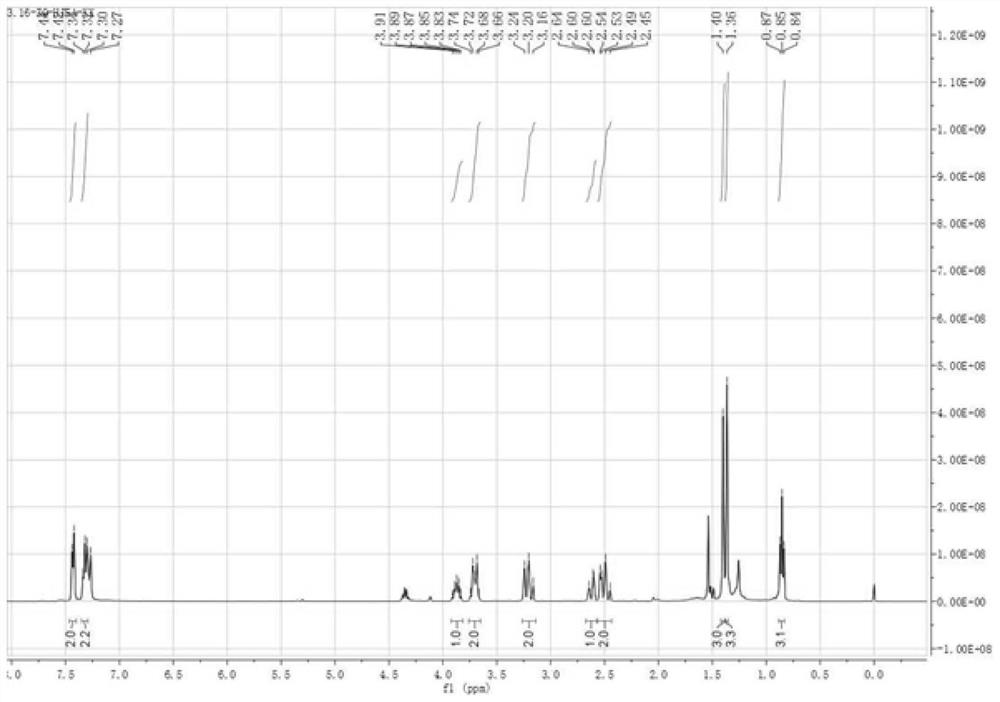
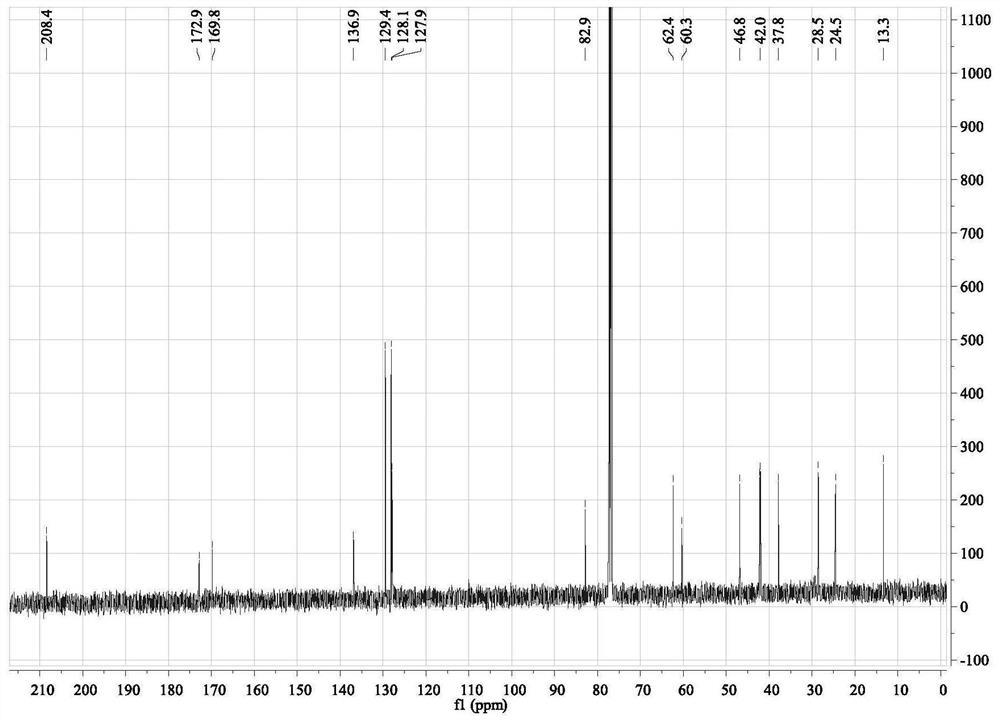
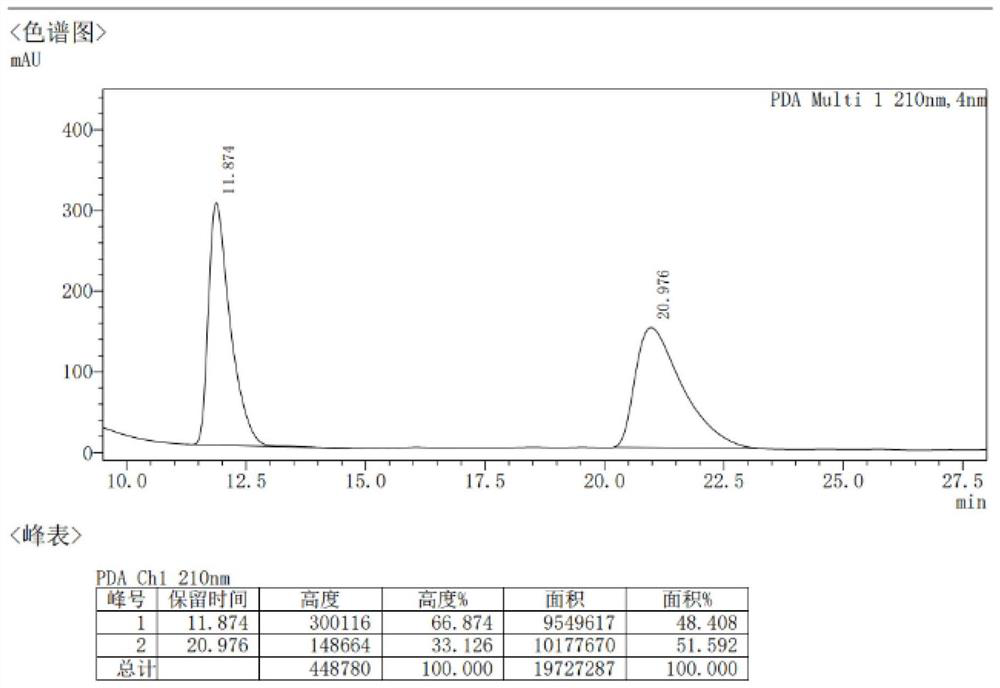
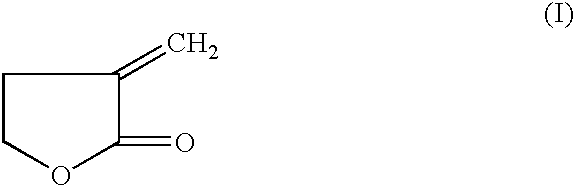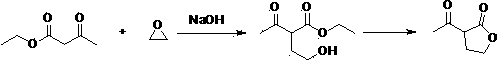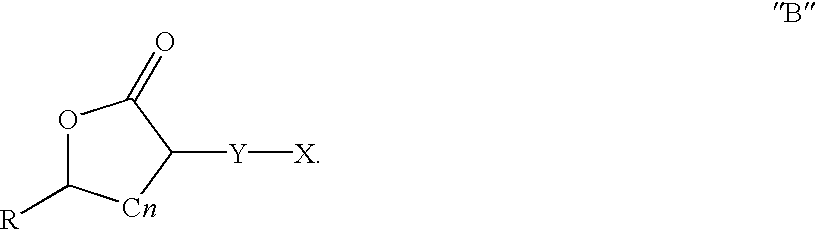Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
40 results about "Butyrolactone I" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Synthetic method for glufosinate ammonium
InactiveCN109232644AMild reaction conditionsEasy to operateGroup 5/15 element organic compoundsPhosphorus tribromideDistillation
The invention relates to a synthetic method for glufosinate ammonium. The synthetic method comprises the following steps: single-bromine substitution, amination, amino protection, chlorination ring opening, Arbuzov reaction and acidizing hydrolysis ammoniation; the single-bromine substitution means triggering alpha-site single-bromine substitution betweengamma-butyrolactone I and bromine under theexistence of catalyst and performing reduced pressure distillation, thereby acquiring a pure intermediate II alpha-bromine-gamma-butyrolactone; phosphorus tribromide is served as the catalyst; amination means triggering amination reaction between alpha-bromine-gamma-butyrolactone II and ammonium hydroxide, and then adding hydrochloric acid and reflowing, thereby acquiring an intermediate IIIalpha-amino-gamma-butyrolactone hydrochloride. The invention has the beneficial effects: 1) low-costgamma-butyrolactone is taken as a raw material, is subjected to single-bromine substitution with bromineand then is subjected to amination reaction with ammonium hydroxide; the adopted raw materials are low-cost and easily acquired; reaction conditions are mild; operation is simple and convenient; safety is high; amplifying production is feasible; reaction yield is high; product purity is high; cost is greatly lowered; the synthetic method is suitable for industrial production.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
Preparation method and application of marine fungi aspergillus terreus butyrolactone compound butyrolactone-I
InactiveCN108245508AEasy to removeImprove antioxidant capacityOrganic active ingredientsNervous disorderDPPHInducer
The invention relates to a preparation method and application of a marine fungi aspergillus terreus butyrolactone compound butyrolactone-I. The invention discloses application of the marine fungi aspergillus terreus butyrolactone compound butyrolactone-I as shown in a formula (I) to preparation of medicaments for resisting to peripheral and neurogenic inflammation and resisting to neurodegenerative diseases. The formula is shown in the description. The invention finds that besides a DPPH free radical, butyrolactone-I can also well remove an ABTS free radical and an OH free radical; the marinefungi aspergillus terreus butyrolactone compound butyrolactone-I has better oxidation resistance and inflammation resistance and has better neuroprotection activity; therefore, the marine fungi aspergillus terreus butyrolactone compound butyrolactone-I has a wide application prospect in the aspect of preparing the medicaments for resisting to peripheral and neurogenic inflammation and resisting toneurodegenerative diseases. Meanwhile, by the preparation method of the butyrolactone-I, which is provided by the invention, the butyrolactone-I can be successfully prepared; the method is simple andeasy to realize large-scale production; culture conditions can also be optimized by addition of an inducer, so that yield of the butyrolactone-I is greatly improved.
Owner:SHENZHEN INST OF GUANGDONG OCEAN UNIV +1
Gamma-butyrolactone compound and pharmaceutical composition thereof
InactiveUS20060079575A1Strong specificityInhibition is effectiveBiocideOrganic chemistryCytotoxicityLung cancer
A γ-butyrolactone compound as shown in Formula (I) and pharmaceutical composition thereof: wherein X═N, O, S, Se; and A and B are selected from substituents having the following formula: wherein R1, R2, R3, R4, and R5 are selected from a hydrogen atom, a halogen atom, a hydroxyl group, a mercapto group, an amino group, an alkoxy group, and a nitro group. The γ-butyrolactone compound and pharmaceutical composition thereof butyrolactone have inhibitory effects on hepatoma, ovarian cancer, breast cancer, lung cancer, malignant glioblastoma or colorectal carcinoma, and are cytotoxic with high specificity to inhibit Paclitaxel-resistant tumour cells at later stage of chemotherapy without any damage on normal cells.
Owner:BUDDHIST TZU CHI GEN HOSPITAL
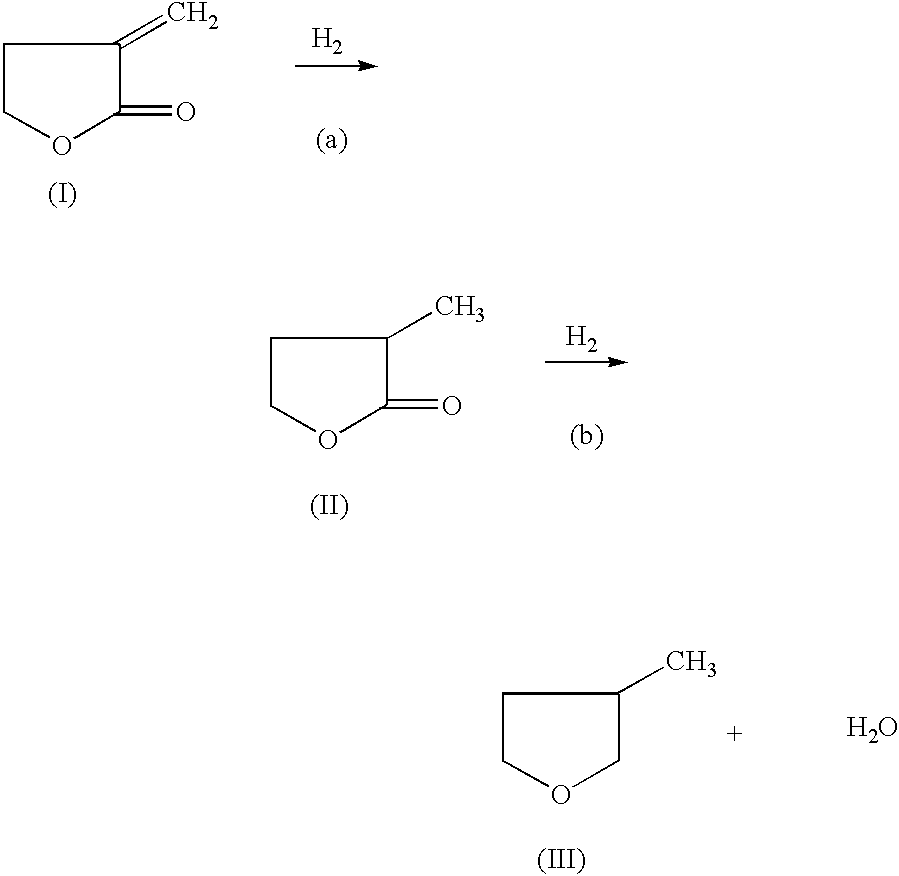
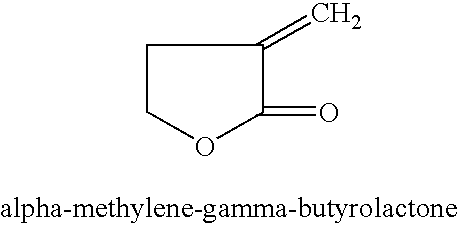
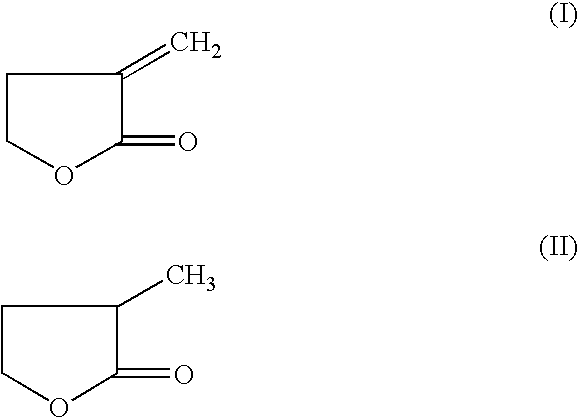
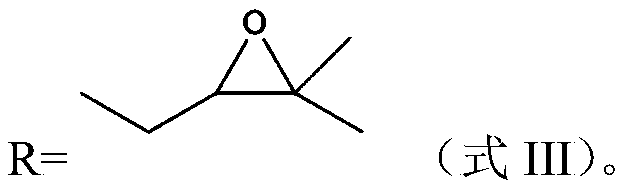
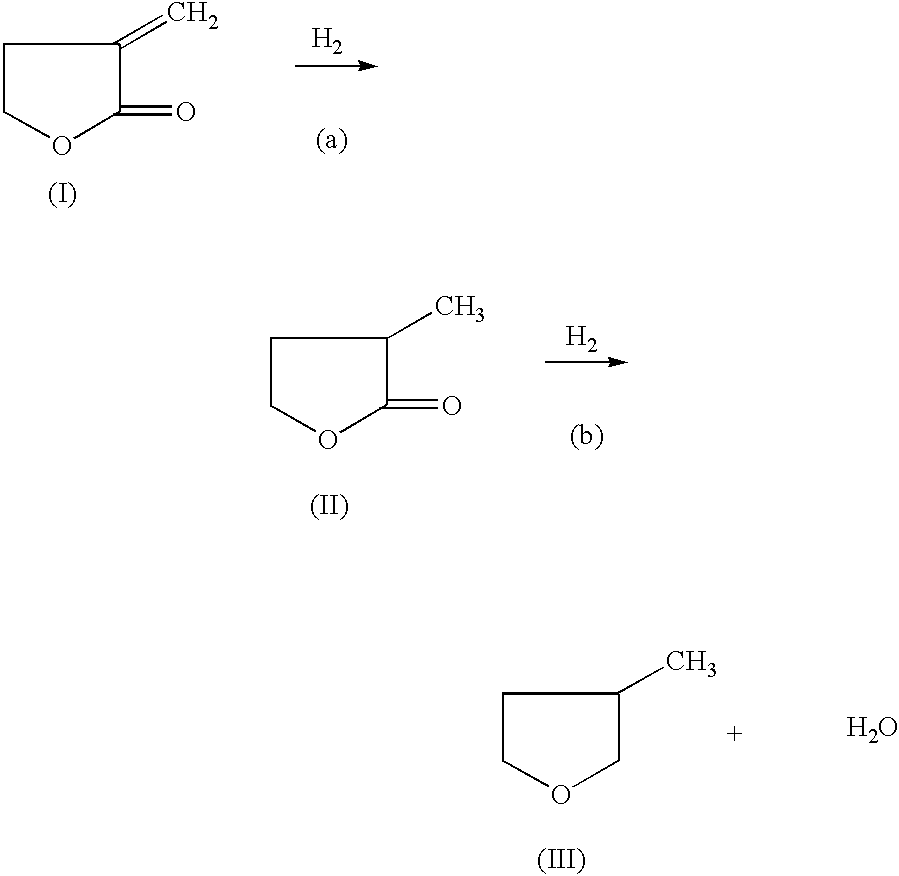
Manufacture of 3-methyl-tetrahydrofuran from alpha-methylene-gamma-butyrolactone in a two step process
InactiveUS6664402B2Enhance physical and chemical functionFunction increaseOrganic chemistryAlcoholHydrogenation process
Disclosed is a two-step, continuous hydrogenation process for the preparation of 3-methyl-tetrahydrofuran from alpha-methylene-gamma-butyrolactone, which comprises a first step of subjecting alpha-methylene-gamma-butyrolactone to hydrogenation to synthesize 2-methyl-gamma-butyrolactone; and the second step of hydrogenating the 2-methyl-gamma-butyrolactone formed in the first step. The above process enables the production of the objective highly-pure 3-methyl-tetrahydrofuran free from alcohol in high efficiency and high conversion through simple production steps.
Owner:INVISTA NORTH AMERICA S A R L
Application of aspergillus terreus secondary metabolite-butyrolactone in preparation of medicament for treating diabetes
InactiveCN109999024AImprove inflammationReduce generationOrganic active ingredientsMetabolism disorderSecondary metaboliteEthyl acetate
The invention provides an application of aspergillus terreus secondary metabolite-butyrolactone in preparation of a medicament for treating diabetes, and belongs to the technical field of microbial medicaments. The aspergillus terreus secondary metabolite-butyrolactone comprises butyrolactone I, wherein the butyrolactone I is extracted according to the following method: fermenting aspergillus terreus OUCMDZ-2739 inoculated in fermentation broth for 25-35 days at 20-30 DEG C; filtering the fermentation broth after fermentation with denim to separate filtrate and mycelium, respectively extracting with ethyl acetate, mixing the obtained ethyl acetate extracts, and concentrating to obtain ethyl acetate solution extract; and further purifying the ethyl acetate solution extract to obtain the butyrolactone I. By the adoption of the application of the aspergillus terreus secondary metabolite-butyrolactone in the preparation of the medicament for treating diabetes, the medicament can inhibit activity of alpha-glucosidase, regulate a composition of intestinal flora, metabolize more short-chain fatty acids, reduce a uric acid level, maintain synthesis of insulin, avoid rising of a blood sugarlevel, and achieve the purpose of relieving type 2 diabetes.
Owner:嘉兴市爵拓科技有限公司
Novel chemo-enzymatic process for the preparation of opticaly enriched beta-benzyl-gamma-butyrolactones
Owner:COUNCIL OF SCI & IND RES
Manufacture of 3-methyl-tetrahydrofuran from alpha-methylene-gamma-butyrolactone in a two step process
InactiveUS20030109724A1Avoid formingIncrease temperatureOrganic chemistryAlcoholHydrogenation process
Disclosed is a two-step, continuous hydrogenation process for the preparation of 3-methyl-tetrahydrofuran from alpha-methylene-gamma-butyrolactone, which comprises a first step of subjecting alpha-methylene-gamma-butyrolactone to hydrogenation to synthesize 2-methyl-gamma-butyrolactone; and the second step of hydrogenating the 2-methyl-gamma-butyrolactone formed in the first step. The above process enables the production of the objective highly-pure 3-methyl-tetrahydrofuran free from alcohol in high efficiency and high conversion through simple production steps.
Owner:INVISTA NORTH AMERICA R L
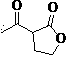
Green synthesis process of alpha-acetyl-gamma-butyrolactone
InactiveCN103360349AReduce pollutionAvoid safety hazardsOrganic chemistryCombinatorial chemistryEthyl acetate
The invention relates to a preparation method of a compound alpha-acetyl-gamma-butyrolactone represented by a formula (I). According to the invention, the compound of the formula (II) and ethyl acetate are subjected to condensation, acidification, and rectification under the existence of calcium oxide, such that the compound represented by the formula (I) is obtained. The compound is an important intermediate used for preparing vitamin and chlorophyll raw materials.
Owner:SHANDONG FANGMING PHARMACEUTICAL CO LTD
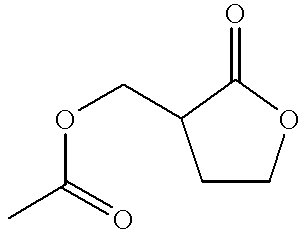
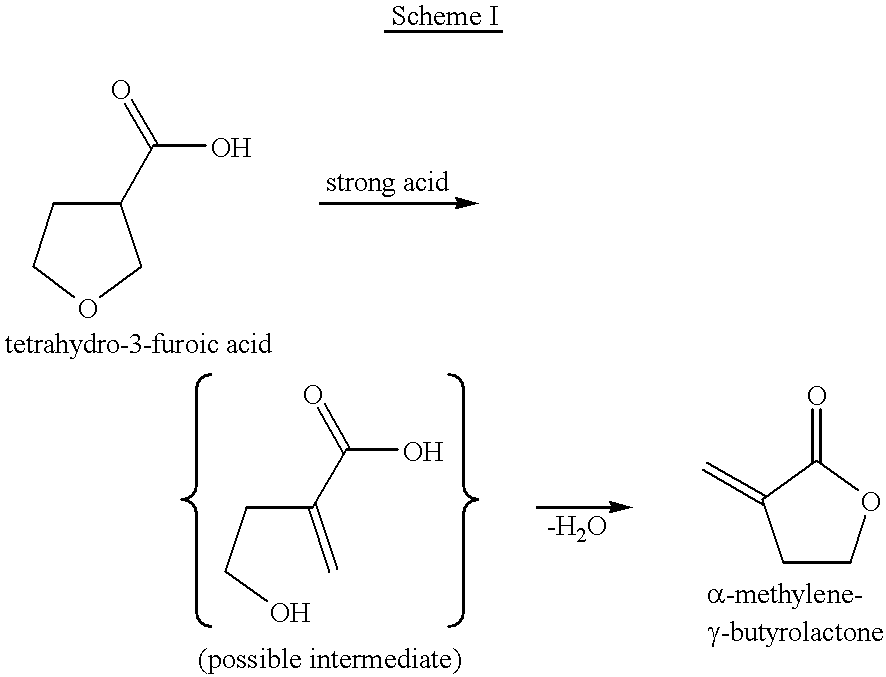
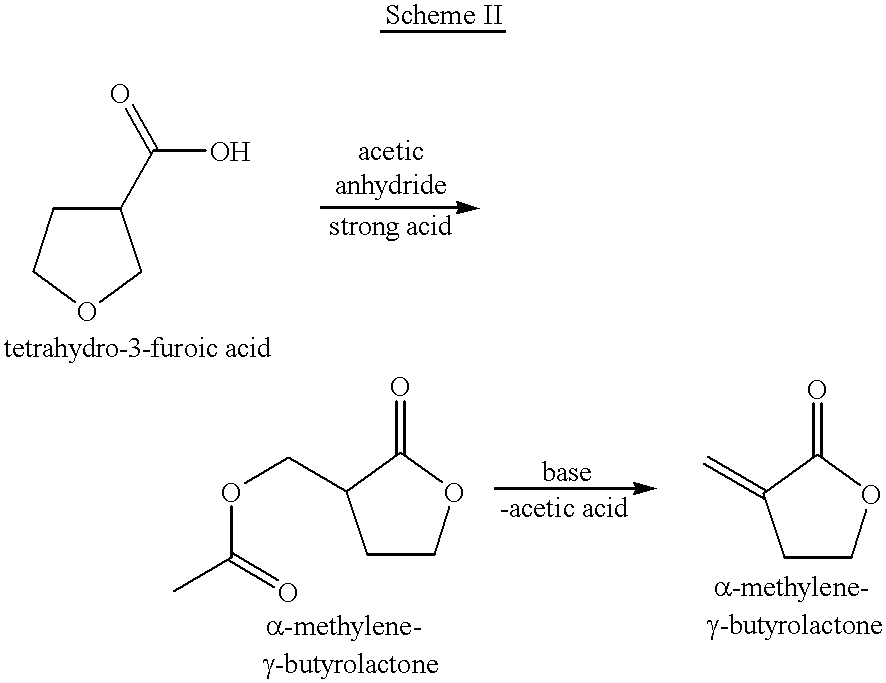
Process for the preparation of alpha-methylene-gamma-butyrolactone and alpha-acetoxymethyl-gamma-butyrolactone
This invention pertains to a process for making alpha-methylene-gamma-butyrolactone by acid-catalyzed rearrangement of tetrahydro-3-furoic acid. In a further embodiment, when tetrahydro-3-furoic acid is treated with acetic anhydride and an acid catalyst, alpha-acetoxymethyl-gamma-butyrolactone is produced in high yield. Under basic conditions, alpha-acetoxymethyl-gamma-butyrolactone can readily formalpha-methylene-gamma-butyrolactone by the elimination of acetic acid. These reactions provide alpha-methylene-gamma-butyrolactone by novel routes which do not require butyrolactone or formaldehyde.
Owner:EI DU PONT DE NEMOURS & CO
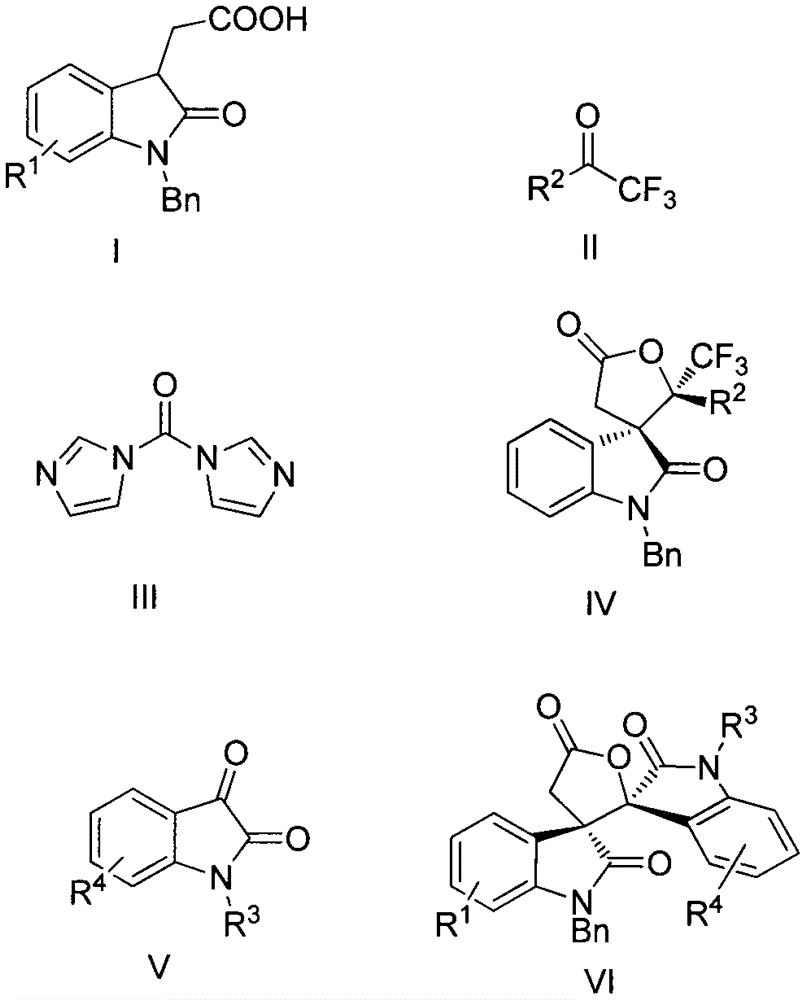
Synthesis method of spiro-oxindole gamma-butyrolactone compound
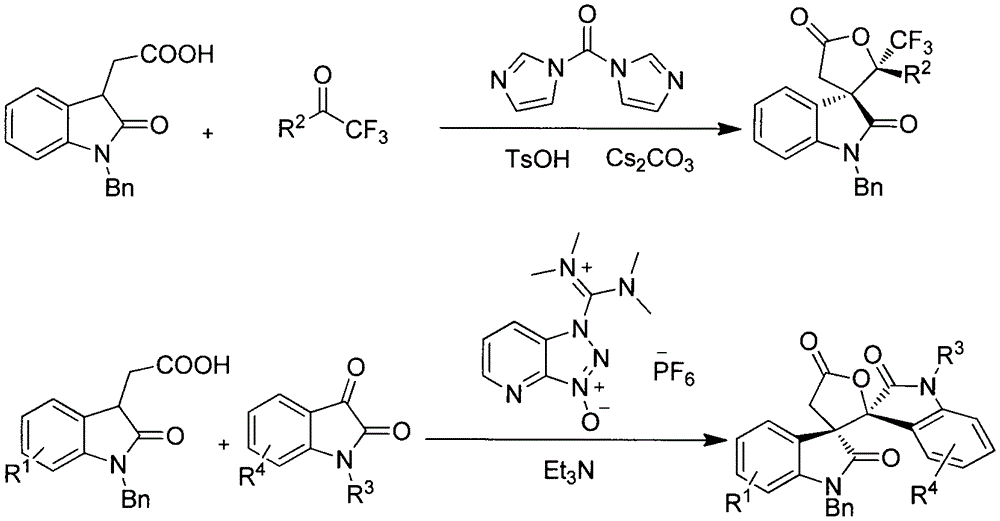
InactiveCN106749295ASimple and fast operationMild reaction conditionsOrganic chemistrySynthesis methodsEvaporation
The invention relates to the field of organic chemistry and in particular relates to a spiro-oxindole gamma-butyrolactone compound IV. The spiro-oxindole gamma-butyrolactone compound IV is prepared by taking an acid shown as a formula I and acetone shown as a formula II as raw materials, and taking dichloromethane as a solvent in the presence of N,N'-carbonyl diimidazole shown as a formula III, p-toluene sulfonic acid and cesium carbonate, reacting at 25 DEG C for 4h, concentrating a reaction solution, eluting by column chromatography, collecting eluting solution parts of all detected products and carrying out rotary evaporation to remove the solvent. The invention further provides a spiro-oxindole gamma-butyrolactone compound VI which is prepared by taking the acid shown as the formula I and N-substituted isatin shown as a formula V as raw materials, and taking tetrahydrofuran as a solvent in the presence of 2-(7-benzotriazole oxide)-N,N,N',N'-tetramethyluronium hexafluorophosphate and triethylamine, reacting at 0 DEG C for 6h, concentrating a reaction solution, eluting by column chromatography, collecting eluting solution parts of all detected products and carrying out rotary evaporation to remove the solvent. A synthesis method has the advantages of relatively good yield, wide substrate applicable range, simplicity and convenience for operation, moderate reaction, convenience for post-treatment and the like.
Owner:CHINA PHARM UNIV
Preparation method of chiral gamma-butyrolactone compounds and derivatives thereof and application thereof
The invention discloses a chiral gamma-butyrolactone compound and a preparation method and an application of a derivative of the chiral gamma-butyrolactone compound. According to the preparation method, an aldehyde compound and 2-furanone are subjected to an organic catalytic asymmetric Michael addition reaction. The invention provides an effective method for combining chiral gamma-butyrolactone with three continuous chiral carbons to obtain the chiral gamma-butyrolactone compound with the yield of 89%, the dr of 9.2:1 and the ee of 99% and the derivative thereof. In addition, a cell activitytest is carried out on the chiral gamma-butyrolactone derivative obtained in the invention, so that the compound has an inhibition effect on proliferation of U251, Saos2, MGC803, 293T and Hela cell lines, and can be used as a broad-spectrum drug in treatment of various tumors.
Owner:INST OF PHARMACY SHANDONG PROV ACAD OF MEDICAL SCI
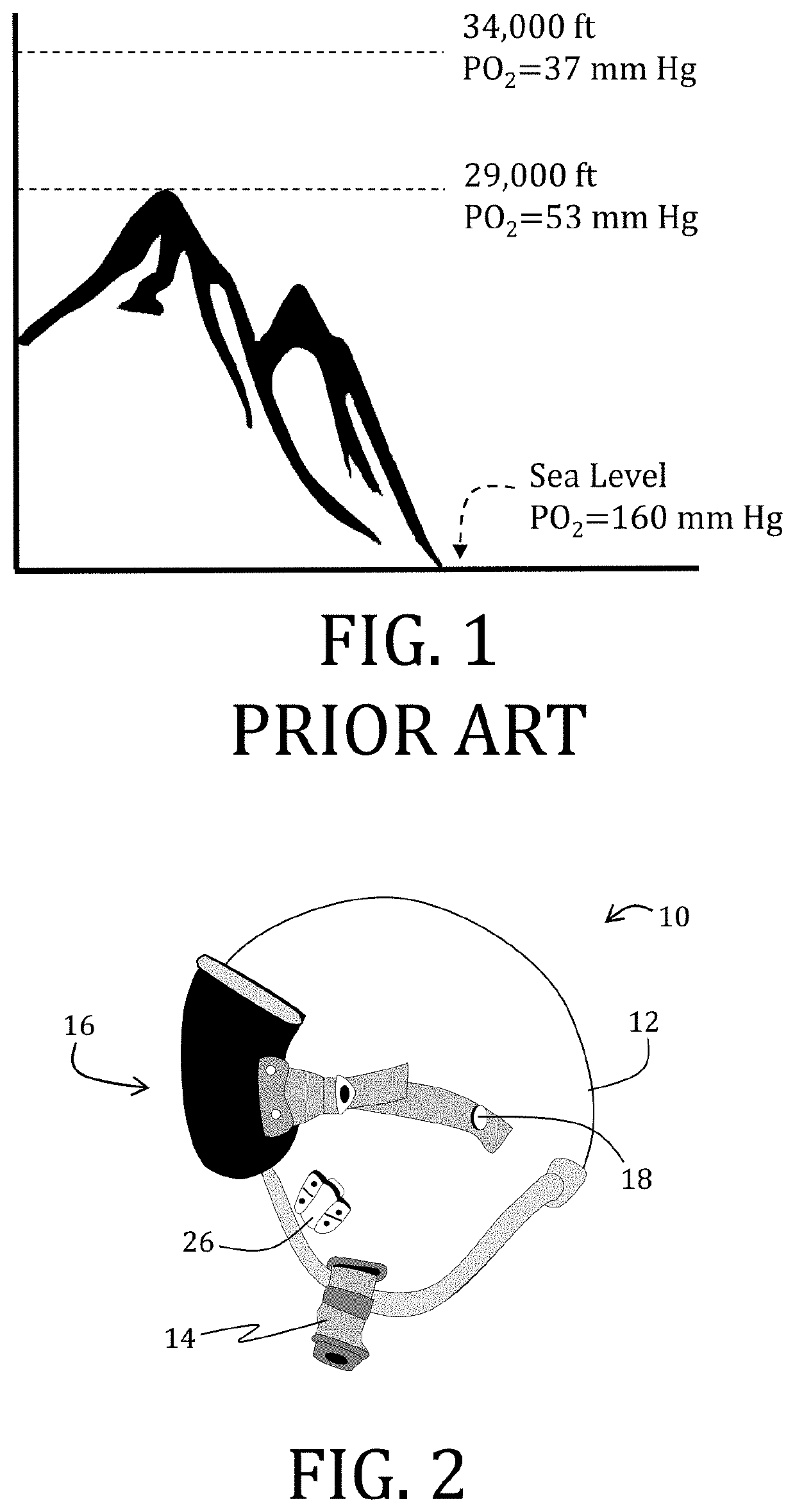
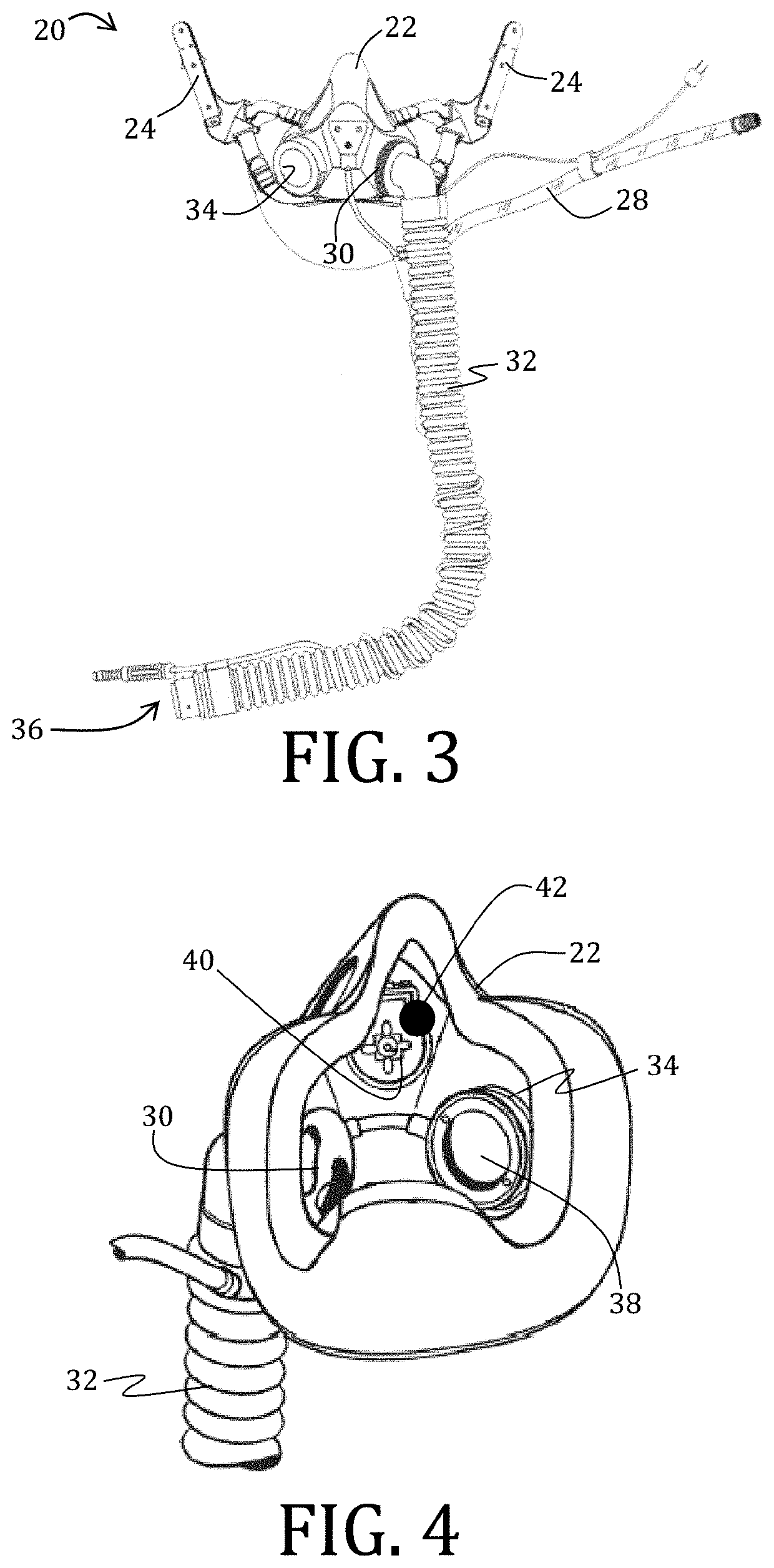
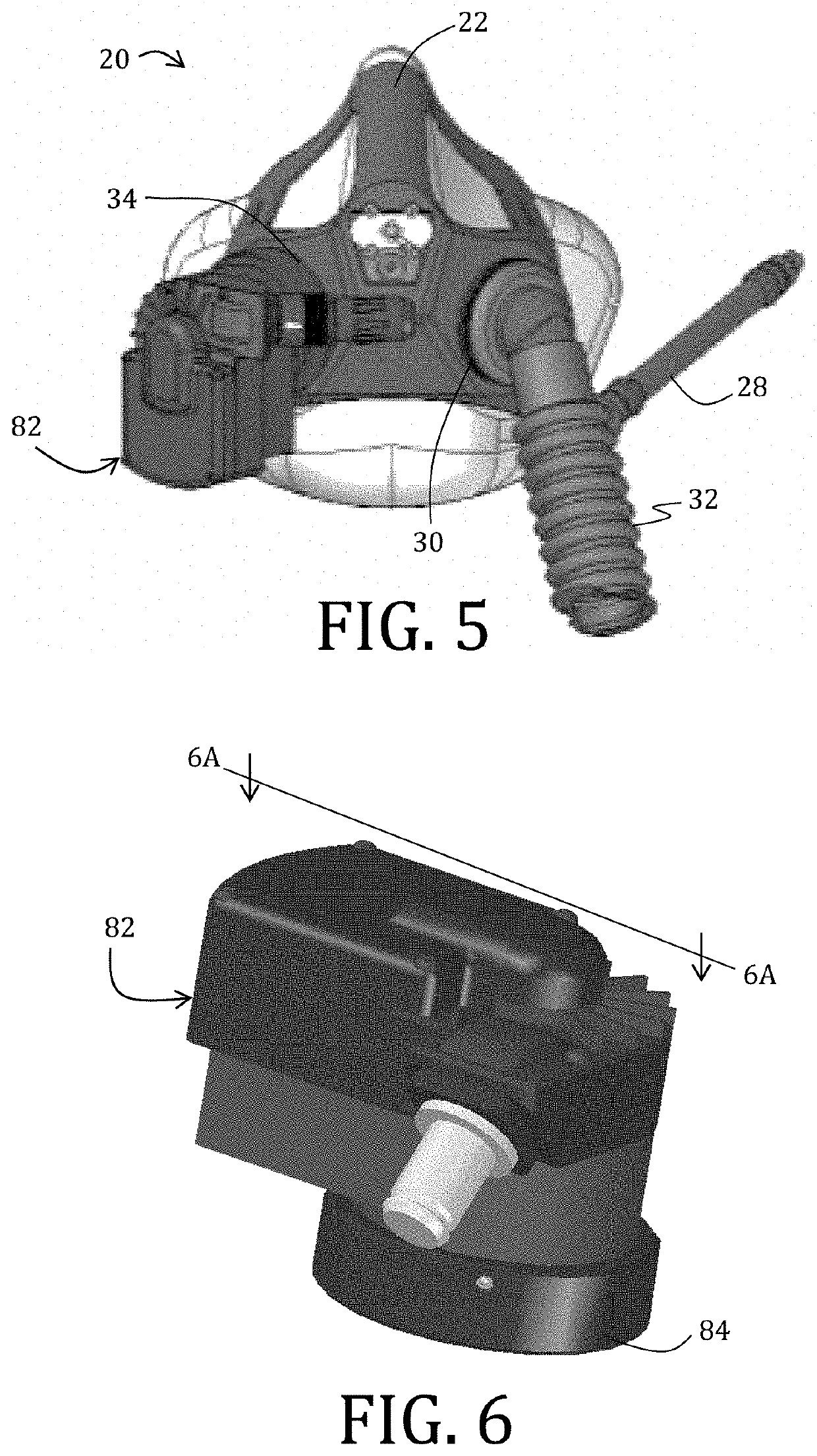
Exhaled breath hypoxia biomarkers
Owner:THE UNITED STATES OF AMERICA AS REPRESETNED BY THE SEC OF THE AIR FORCE
A kind of method for continuously synthesizing n-methylpyrrolidone and n-ethylpyrrolidone
ActiveCN107474003BShort synthesis reaction timeImprove efficiencyOrganic chemistryPyrrolidinonesEthyl group
A method for continuously synthesizing N-methylpyrrolidone and N-ethylpyrrolidone, the method is carried out in a microreactor, and gamma-butyrolactone solution and corresponding alkylamine solution are continuously passed through a microreactor to synthesize N-methylpyrrolidone and N-ethylpyrrolidone. Wherein, the microreactor includes a reaction section and a reaction suppression section, and the reaction mixture is carried out under the condition that the residence time of the reaction mixture is 1 to 30 minutes, and wherein the γ-butyrolactone solution and the corresponding alkylamine solution Both use ethylene glycol as a solvent, the molar ratio of alkylamine and γ-butyrolactone in the reaction mixture is 1.0-1.6, and the concentration of the γ-butyrolactone solution is 0.5-2 mol / L. The invention can shorten the synthesis process time of N-methylpyrrolidone and N-ethylpyrrolidone from several hours to within 30 minutes, and meanwhile, the yield of the product reaches more than 90%.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Preparation method of alpha-acetyl-gamma-butyrolactone
ActiveCN111620844AEradicationEase of mass productionOrganic chemistryOrganic synthesisCombinatorial chemistry
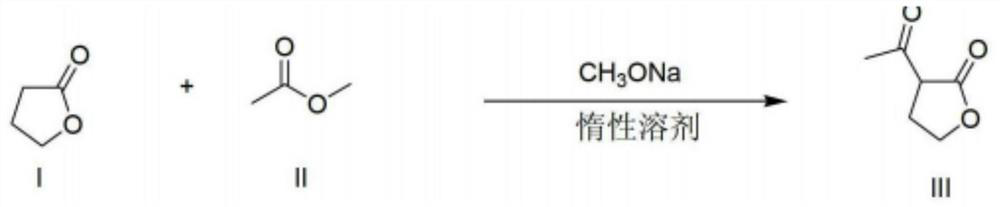
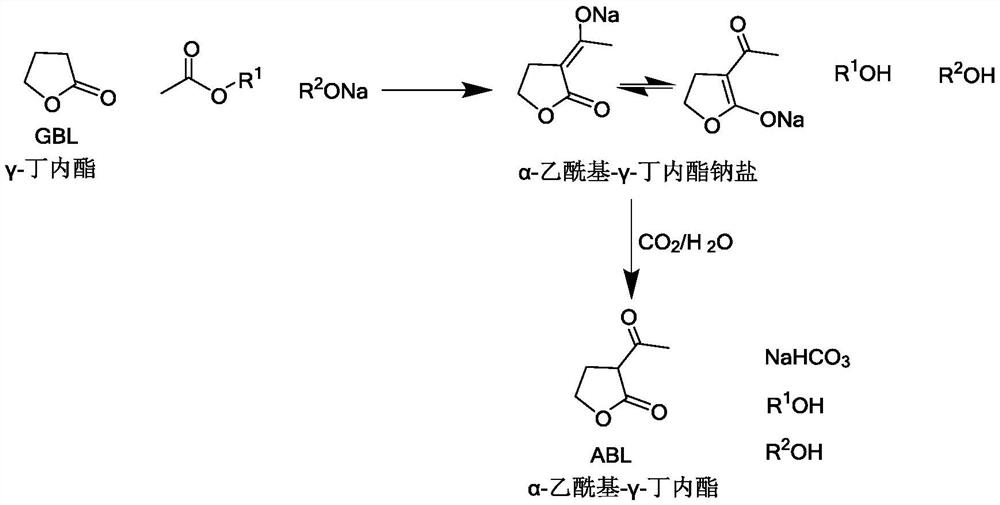
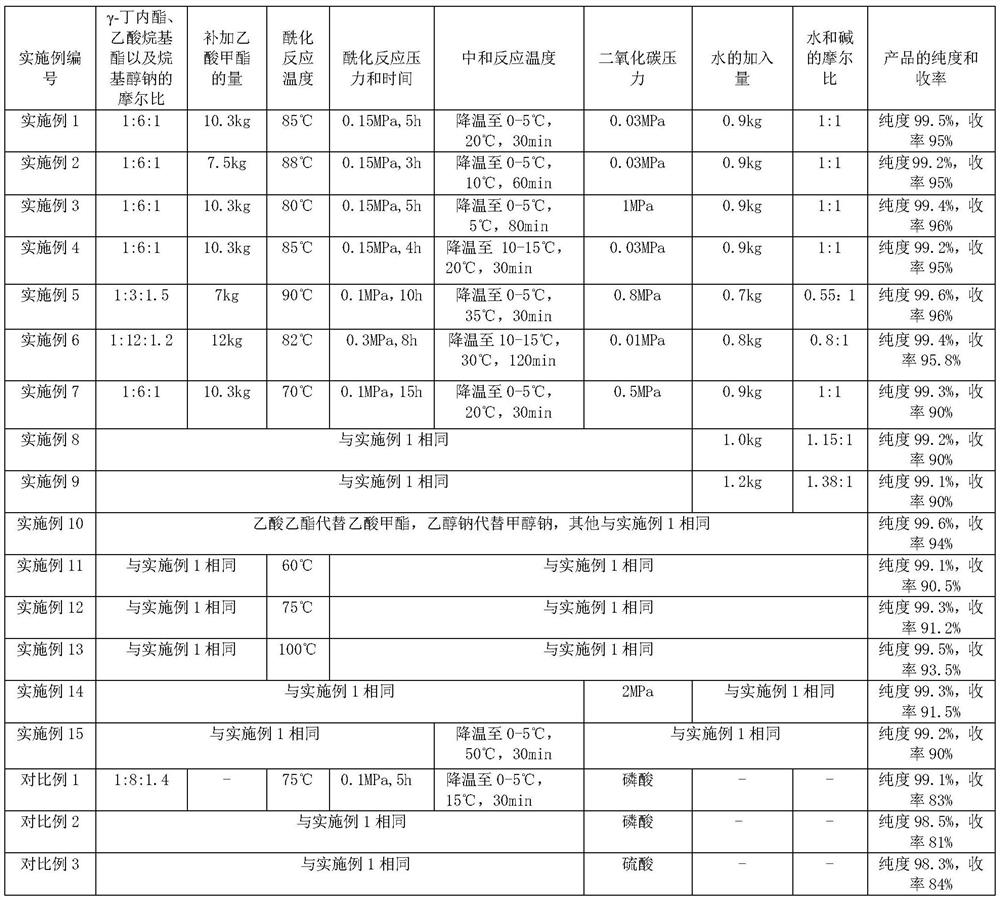
The invention relates to the field of organic synthesis, and discloses a preparation method of alpha-acetyl-gamma-butyrolactone. The method comprises the following steps: (1) gamma-butyrolactone, CH3COOR1 and R2ONa are subjected to acylation reaction to obtain a material containing alpha-acetyl-gamma-butyrolactone sodium salt, and R1 and R2 are respectively independently C1-C4 alkyl; and (2) in the presence of water, the material containing the alpha-acetyl-gamma-butyrolactone sodium salt is enabled to be in contact with CO2 gas to generate neutralization reaction. The method also has the advantage of higher yield under the condition of ensuring safety, and provides convenience for large-scale production of alpha-acetyl-gamma-butyrolactone.
Owner:江西天新药业股份有限公司 +1
Process for the preparation of gamma-butyrolactones
Owner:LONZA LTD
Preparation method of racemic beta-aryl-gamma-butyrolactone compound
The invention discloses a preparation method of a racemic beta-aryl-gamma-butyrolactone compound. According to the preparation method, an arylboronic acid compound and gamma, gamma-disubstituted furanone are subjected to an organic catalytic asymmetric Michael addition reaction. According to the efficient preparation method provided by the invention, the gamma-butyrolactone compound reacts with aryl, and the racemic beta-aryl-gamma-butyrolactone compound with a yield as high as 98% is obtained. In addition, an intermediate is provided for preparation of a leukemia stem cell inhibitor with moreeffective activity.
Owner:INST OF PHARMACY SHANDONG PROV ACAD OF MEDICAL SCI
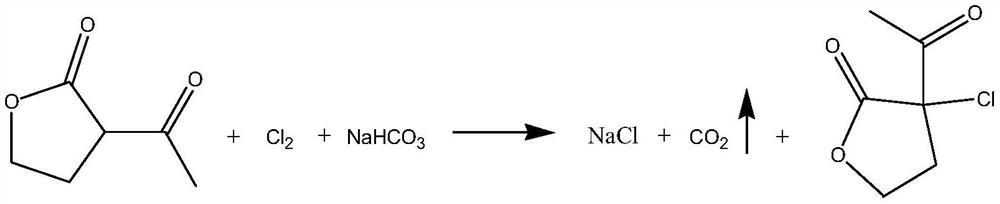
Method for preparing alpha-chloro-alpha-acetyl-gamma-butyrolactone
InactiveCN111018811AEasy to operateMild reaction conditionsOrganic chemistryOrganic synthesisCombinatorial chemistry
The invention relates to the field of organic synthesis, and particularly discloses a method for preparing alpha-chloro-alpha-acetyl-gamma-butyrolactone. The preparation method comprises the followingsteps: (1) in the presence of a solvent, carrying out a salt forming reaction on alpha-acetyl-gamma-butyrolactone and at least one inorganic alkaline substance selected from NaOH, KOH, K2CO3 and Na2CO3 until a precipitate is generated, and then dissolving the precipitate to obtain a first solution; (2) carrying out a contact reaction on the first solution and chlorine to obtain a second solution;and (3) layering the second solution so as to obtain the alpha-chloro-alpha-acetyl-gamma-butyrolactone. The method has the advantages of simple operation, environmental protection, mild reaction conditions, and important practical significance.
Owner:江西天新药业股份有限公司
Application of liquid sodium methoxide in synthesis of alpha-acetyl-gamma-butyrolactone and synthesis method of alpha-acetyl-gamma-butyrolactone
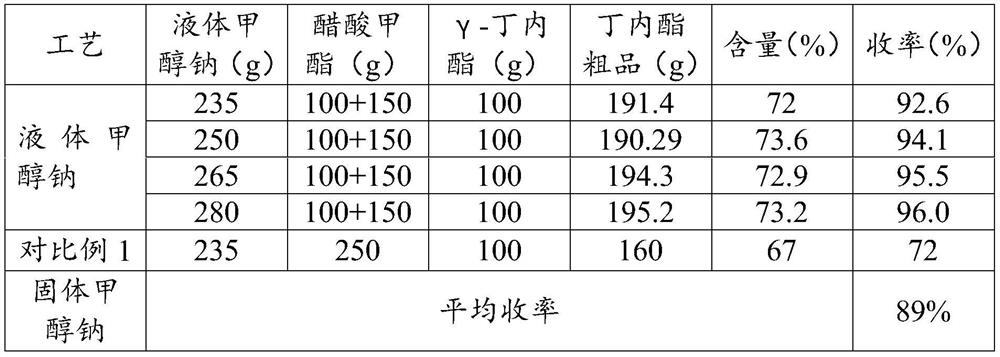
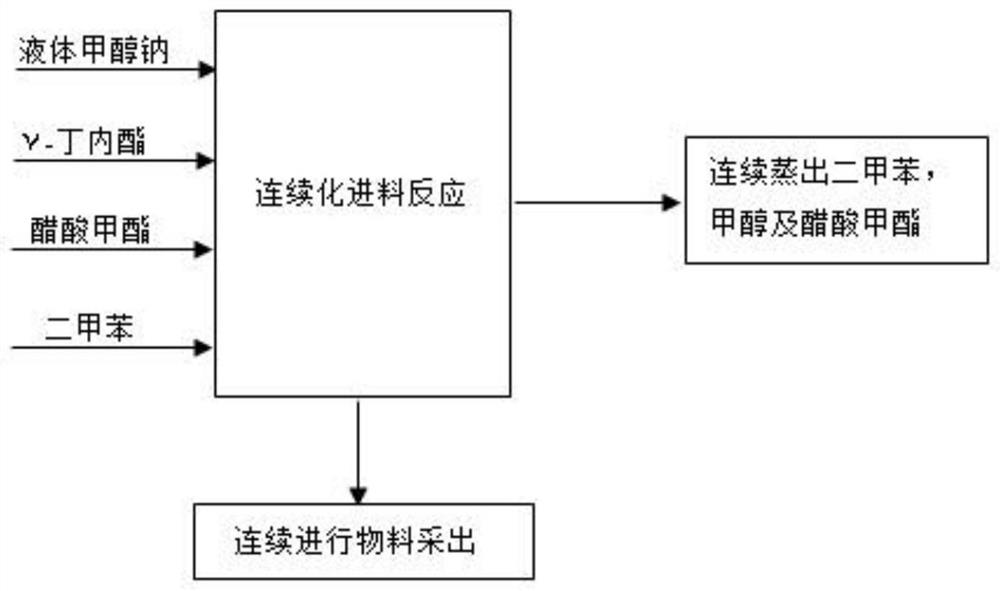
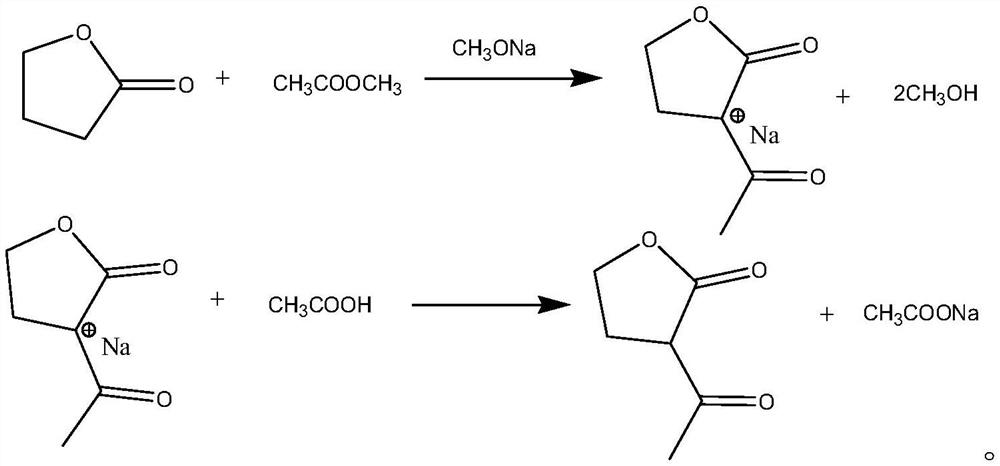
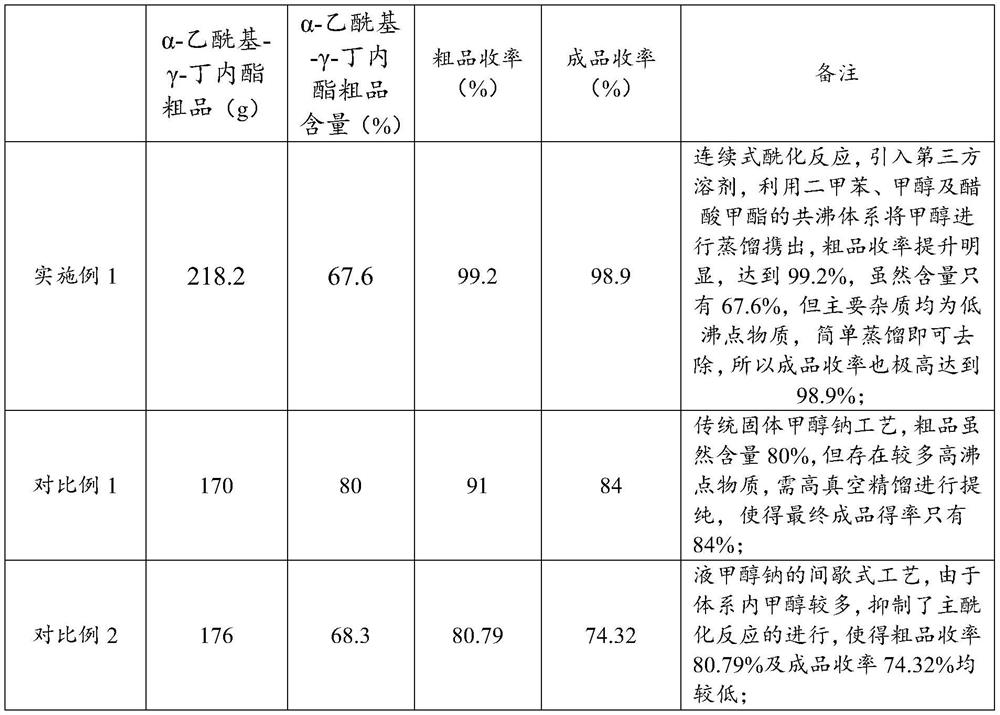
ActiveCN112759566ARealize feeding liquefactionAchieve airtightnessOrganic chemistrySodium methoxideAcetic acid
The invention provides application of liquid sodium methoxide in synthesis of alpha-acetyl-gamma-butyrolactone and a synthesis method of the alpha-acetyl-gamma-butyrolactone, and relates to the technical field of organic synthesis. The synthesis method of the alpha-acetyl-gamma-butyrolactone comprises the following steps: (a) performing pre-acylation reaction on an acetate compound and gamma-butyrolactone; (b) adding liquid sodium methoxide into the reaction liquid in the step (a) to carry out mixed reaction; (c) after the reaction in the step (b) is finished, concentrating and collecting methanol, and transferring the concentrated reaction liquid into an acylation kettle; (d) supplementing the acetate compound into the acylation kettle for acylation reaction; (e) performing neutralizing, filtering and concentrating to obtain an alpha-acetyl-gamma-butyrolactone crude product. According to the method, liquid sodium methoxide is used for replacing solid sodium methoxide for acylation synthesis, so that liquification and sealing of feeding are realized, the on-site feeding risk is reduced, and the synthesis yield is increased to 96% or above.
Owner:江苏兄弟维生素有限公司
Preparation method and application of alpha-acetyl-gamma-butyrolactone
PendingCN114195745AEliminate potential safety hazardsRemove inhibitionOrganic chemistryCombinatorial chemistryMedicinal chemistry
The invention provides a preparation method and application of alpha-acetyl-gamma-butyrolactone, and relates to the technical field of chemical engineering, and the preparation method comprises the following steps: gamma-butyrolactone, an acylation reagent, an alkaline reagent and a benzene reagent are mixed and then subjected to an acylation reaction, during the acylation reaction, the acylation reagent, the benzene reagent and byproducts are azeotropic to remove the byproducts, and the alpha-acetyl-gamma-butyrolactone is obtained. Carrying out acid neutralization to obtain alpha-acetyl-gamma-butyrolactone; wherein the alkaline reagent comprises an alkoxide solution; the by-product is a by-product generated by an alkaline reagent. The preparation method provided by the invention solves the technical problems that a solid alkaline reagent brings potential safety hazards to manual feeding, the acylation reaction yield is low and the product purification operation is complicated, and achieves the technical effects of eliminating the potential safety hazards of solid feeding, improving the acylation reaction yield and being simple in product purification operation.
Owner:江苏兄弟维生素有限公司
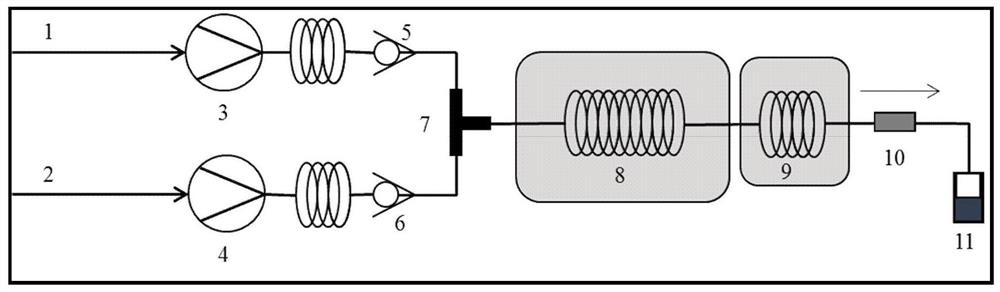
Catalyst for preparing gamma-butyrolactone through maleic anhydride liquid phase hydrogenation, preparation method and application of catalyst, and method for preparing gamma-butyrolactone
InactiveCN111097427AHigh activityHigh selectivityOrganic chemistryHeterogenous catalyst chemical elementsPtru catalystFluid phase
The invention belongs to the technical field of catalysts, and discloses a catalyst for preparing gamma-butyrolactone through maleic anhydride liquid phase hydrogenation, a preparation method and application of the catalyst, and a method for preparing gamma-butyrolactone. The catalyst comprises the following components in percentage by weight: 20-70wt% of CuO, 25-75wt% of ZnO, 25-15wt% of SnO and2-8wt% of SiO2. The catalyst has high activity and selectivity, the conversion rate of maleic anhydride is greater than or equal to 95%, and the selectivity of gamma-butyrolactone is greater than or equal to 90%.
Owner:CHINA PETROLEUM & CHEM CORP +1
Preparation and use of gamma-butyrolactones as cross-linking agents
The rate of aminolysis of butyrolactones is predictably adjusted by attaching a substituent having a known field effect value (F) to the alpha position before reacting the substituted buytrolactone with an amine. The aminolysis product is a gamma-hydroxy amide. The resulting materials are useful as the cross-linking agents in a variety of coatings and coatings processes.
Owner:EI DU PONT DE NEMOURS & CO
The preparation method of Pisicani hydrochloride intermediate
The present invention provides a preparation method of Piscicanide hydrochloride intermediate, which is characterized in that it comprises: Step 1, butyrolactam (I) and γ-butyrolactone (II) undergo polymerization reaction under strong base and predetermined conditions Generate N-(3-carboxypropyl) butyrolactam (Ⅲ); Step 2, N-(3-carboxypropyl) butyrolactam (Ⅲ) is cyclized at high temperature in monoisopropyl malonate solvent and Condensation with malonate monoester salt to generate 7α-double condensed pyrrolidine-acetate; a better choice is to generate 7α-double condensed pyrrolidine-acetate isopropyl ester (Ⅴ). Step 3, 7α-biscondensed pyrrolidine-acetate is hydrolyzed in acid to generate the corresponding pharmaceutically acceptable salt. The preparation method of the invention avoids expensive or difficult-to-obtain raw materials, avoids the use of liquid ammonia in the synthesis process, reduces reaction steps, is simple to operate, has few wastes and high yield.
Owner:HANCHEM BIOPHARM TECH
A kind of preparation method of clam oligopeptide
ActiveCN110713518BHigh extraction rateImprove biological activityPeptide preparation methodsFermentationEnzymatic hydrolysisAlkaline proteinase
Owner:ZHEJIANG OCEAN UNIV
Preparation method of chiral bicyclic gamma-butyrolactone compound
The invention discloses a preparation method of a chiral bicyclic gamma-butyrolactone compound, the chiral bicyclic gamma-butyrolactone compound is a main skeleton of many compounds and drug molecules and is an important active intermediate, for example, a natural product podophyllotoxin and a derivative thereof are widely applied to clinical treatment as an antitumor drug; wherein the vorapaxar sulfate serving as a marketed drug is also used as an initiative oral PAR-1 inhibitor. According to the present invention, through the double Michael addition reaction of gamma-dimethyl furanone and alpha,beta-unsaturated ketone catalyzed by (1S,2S)-1, 2-diphenylethylenediamine, so that the functional chiral bicyclic gamma-butyrolactone compound with the yield of 80%, the stereoselectivity of more than 20:1 and the higher value can be rapidly obtained through the one-step reaction. The method and the thought are provided for the synthesis of the drug active intermediate, so that synthesis difficulty of the compound in the current medicine research and development field is solved.
Owner:SHANDONG FIRST MEDICAL UNIV & SHANDONG ACADEMY OF MEDICAL SCI
Preparation method of clam oligopeptides
ActiveCN110713518AHigh extraction rateImprove biological activityPeptide preparation methodsFermentationAlkaline proteaseOligopeptide
The invention relates to the technical field of polypeptide extraction from clams. In order to further improve the extraction rate of the clam oligopeptides, the invention provides an extraction method of the clam oligopeptides. A binary system obtained by compounding ionic liquid EMIMBF4 and gamma-butyrolactone according to a volume ratio of 1:1 is applied to the preparation of the clam oligopeptides; a pulse electric field is applied at a low temperature for decomposing internal organ tissues of the clams; then, enzymolysis, separation and purification are performed under an optimized enzymolysis condition; and the clam oligopeptides are obtained. The preparation method of the clam oligopeptides has the advantages that a compounding solvent prepared by mixing the ionic liquid EMIMBF4 andthe gamma-butyrolactone according to a ratio of 1:1 is added; low-temperature cooperative pulse electric field treatment is performed; then, enzymolysis is performed by using alkaline proteases; theextraction rate of the obtained clam oligopeptides is improved; the bioactivity is improved to a certain degree; and the degreasing process of 0.1 mol / L sodium hydroxide solution is omitted.
Owner:ZHEJIANG OCEAN UNIV
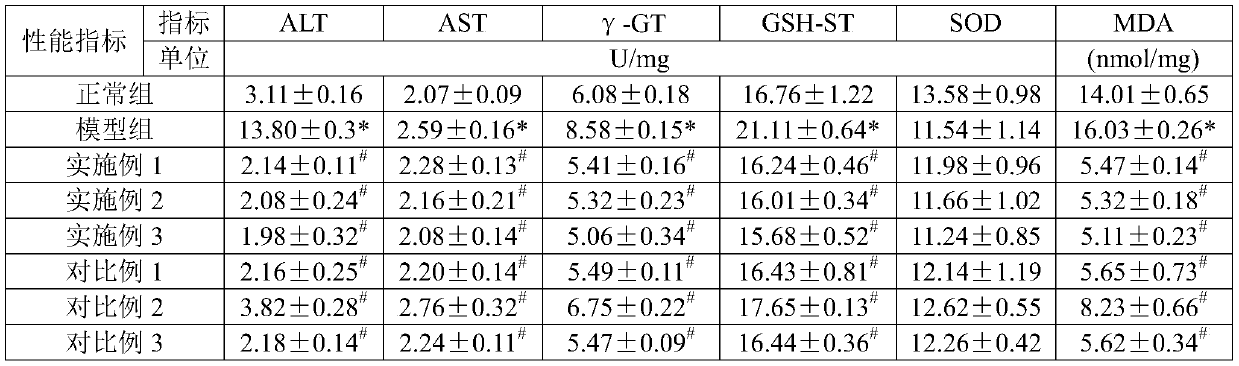
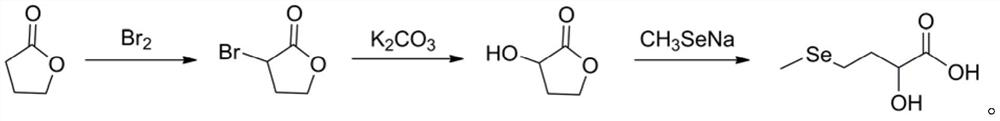
Preparation method of DL-hydroxy selenomethionine
The invention belongs to the field of preparation of organic compounds. The invention provides a preparation method of DL-hydroxy selenomethionine. The method is characterized in that gamma-butyrolactone is used as a raw material, alpha-hydroxyl-gamma-butyrolactone is synthesized through alpha-bromination and hydroxylation, and then the alpha-hydroxyl-gamma-butyrolactone reacts with sodium methyl selenol to obtain the DL-hydroxyl selenomethionine. The method has the advantages of easily available raw materials, mild reaction conditions and low cost, and is suitable for large-scale preparation of DL-hydroxyselenomethionine.
Owner:FUJIAN INST OF RES ON THE STRUCTURE OF MATTER CHINESE ACAD OF SCI
A kind of chiral gamma-butyrolactone derivative and its synthesis method and application
ActiveCN111196791BEnhanced inhibitory effectEasy to prepareOrganic chemistryAntineoplastic agentsOncologyHuman breast
The invention discloses a chiral γ-butyrolactone derivative, a synthesis method and application thereof. Its structure is shown in formula (1); wherein, Ar 1 、Ar 2 and Ar 3 Each independently is phenyl, halogenated phenyl, nitro-substituted phenyl, C 1~4 Alkyl substituted phenyl, C 1~4 Alkoxy substituted phenyl, C 1~4 Haloalkyl is substituted for phenyl, naphthyl or halonaphthyl. The derivatives described in the present invention have a novel structure, and have good anti-cancer effects, especially for human breast cancer cells (MCF-7) and human lung adenocarcinoma cells (A549), and have good inhibitory effects, It exhibits good anti-breast cancer and lung cancer cell effects, and can be prepared as an anti-breast cancer and lung cancer drug for application; in addition, the preparation method of the compound is simple, using cheap and easy-to-obtain compounds as raw materials, and has mild reaction conditions, The invention has beneficial effects such as few reaction steps, fast reaction, less waste generated, simple and safe operation, high atom economy, high selectivity, and high yield.
Owner:SUN YAT SEN UNIV
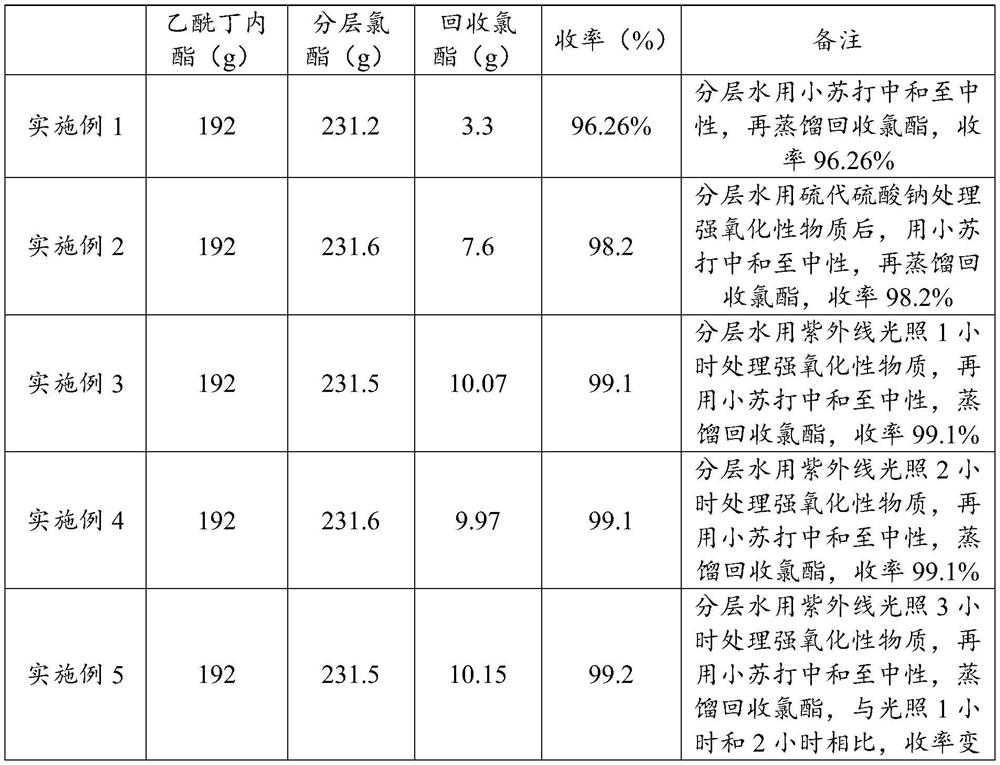
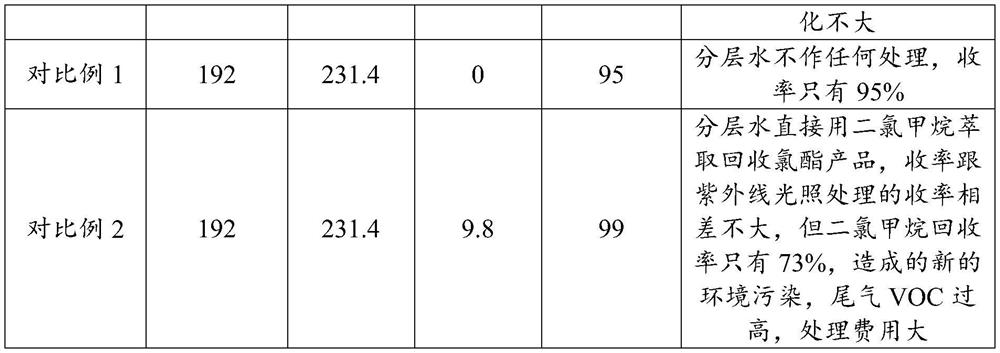
Recovery method and application of alpha-chloro-alpha-acetyl-gamma-butyrolactone layered water
PendingCN114181178AEasy to recycleTroubleshoot poor performanceOrganic chemistryDistillationDesalination
The invention provides a recovery method and application of alpha-chloro-alpha-acetyl-gamma-butyrolactone layered water, and relates to the technical field of chemical engineering. The recovery method comprises the following steps: neutralizing the alpha-chloro-alpha-acetyl-gamma-butyrolactone layered water with alkali, and then evaporating water; and the recovered alpha-chloro-alpha-acetyl-gamma-butyrolactone is obtained by using the method disclosed by the invention. The method provided by the invention solves the technical problem of unsmooth operation of environmental protection facilities caused by viscous substances generated when the alpha-chloro-alpha-acetyl-gamma-butyrolactone layered water is used as wastewater for distillation desalination treatment, and achieves the purpose of recovering alpha-chloro-alpha-acetyl-gamma-butyrolactone from the layered water. The economic benefit is improved; and the operation efficiency of environmental protection facilities is improved.
Owner:江苏兄弟维生素有限公司
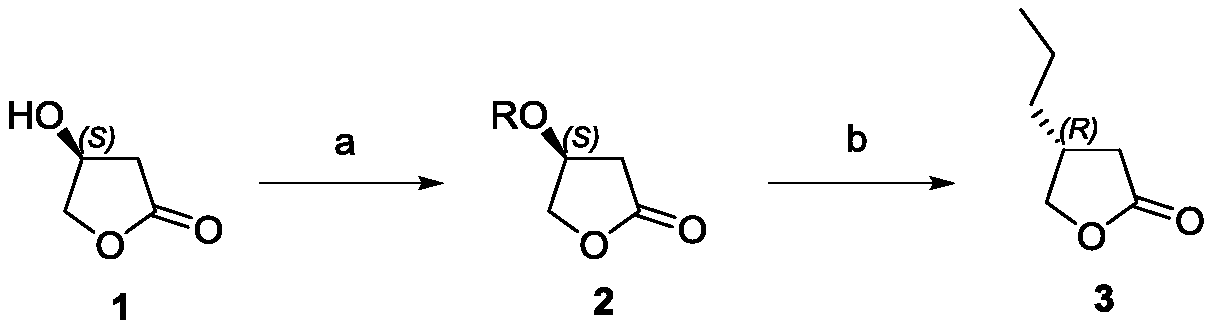
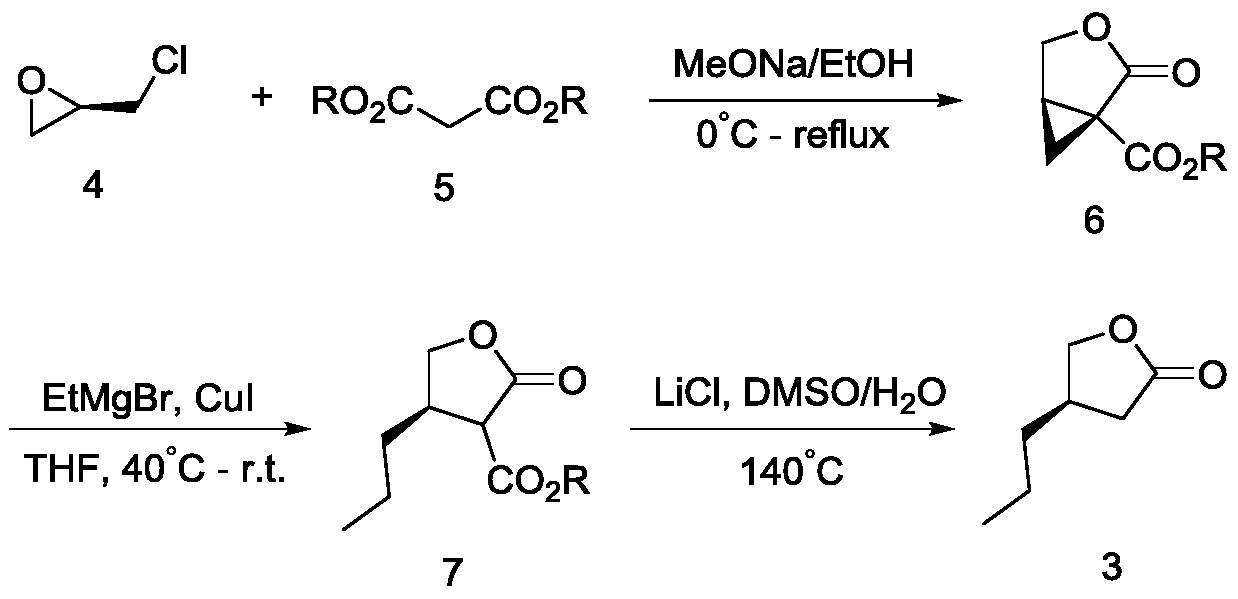
A preparation method of (r)-3-propyl-γ-butyrolactone
ActiveCN108530402BSimple process routeMild reaction conditionsOrganic chemistryPtru catalystGrignard reagent
The invention discloses a preparation method of (R)-3-propyl-gamma-butyrolactone. The method comprises the following steps: with (S)-3-hydroxyl-gamma-butyrolactone as an initial raw material, activating hydroxyl by using sulfonate, reacting the hydroxyl-activated compound with a Grignard reagent in the presence of a copper catalyst, a cocatalyst and a nitrogen-containing compound to generate (R)-3-propyl-gamma-butyrolactone. The preparation method has the advantages that the process route is simple, the raw materials are cheap and easy to obtain, the synthesis route is short, the reaction conditions are mild, the operation is simple and convenient, the yield is high, and the stereoselectivity is good, the problems of the prior art that chiral separation and chiral column separation are needed in reaction, the yield is low and the chemical selectivity is poor are solved, and an important value is provided for the process research of brivaracetam.
Owner:ZHEJIANG UNIV OF TECH
Chiral bicyclic gamma-butyrolactone compound and application thereof
The invention discloses a chiral bicyclic gamma-butyrolactone compound and application thereof, the chiral bicyclic gamma-butyrolactone compound is a main skeleton of many compounds and drug molecules, and is an important active intermediate, for example, a natural product podophyllotoxin and a derivative thereof are widely applied to clinical treatment as an antitumor drug; the vorapaxar sulfate serving as a marketed drug is also used as an initiative oral PAR-1 inhibitor. According to the preparation method disclosed by the invention, through double Michael addition reaction of gamma-dimethyl furanone and alpha, beta-unsaturated ketone catalyzed by (1S, 2S)-1, 2-diphenylethylenediamine, functional products are rapidly obtained through one-step reaction, the yield is as high as 80%, and the stereoselectivity is gt; the chiral dicyclic gamma-butyrolactone compound with a specific molecular weight of 20: 1 and a relatively high er value is prepared, then the chiral dicyclic gamma-butyrolactone compound disclosed by the invention is subjected to a cell viability test, and the tested compound has a certain effect of inhibiting tumor cell viability on U87MG, HCT116, SH-SY-5Y, Kyse-30, MV4-1 and MCF-7 cell lines.
Owner:SHANDONG FIRST MEDICAL UNIV & SHANDONG ACADEMY OF MEDICAL SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com