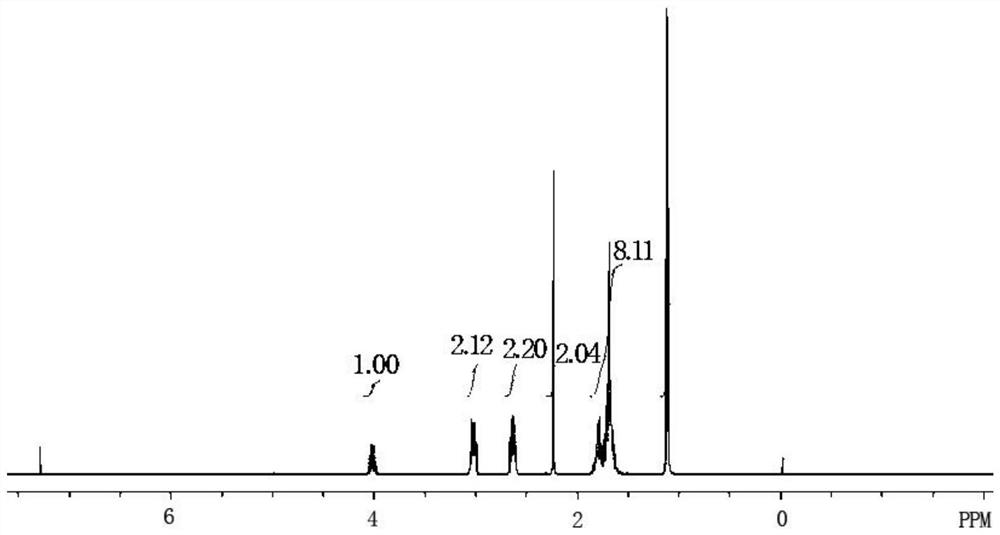
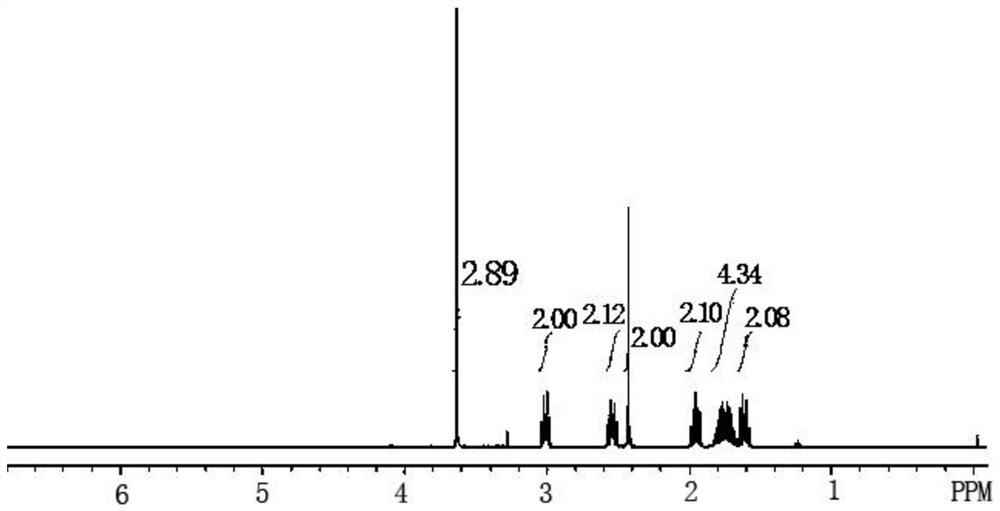
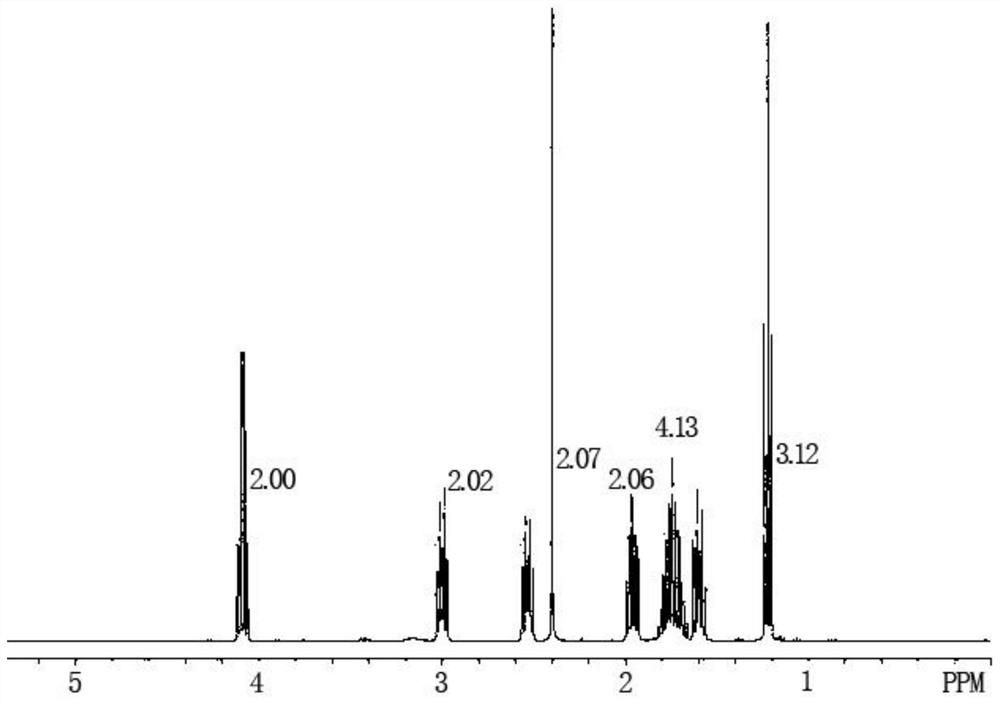
The preparation method of Pisicani hydrochloride intermediate
A technology of piricanib hydrochloride and an intermediate, applied in the field of medicine, can solve the problems of low yield of 8-acetonitrile-based bicyclopyrrolidine, unsuitable for quantitative production, instability of bicyclopyrrolidine and the like, and achieve stable product quality and cost. Low, easy-to-use effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Preparation of N-(3-carboxypropyl)butyrolactam (Ⅲ)
[0045] Add 18.4g (0.8mol) of sodium metal and 86.0g (1.0mol) of γ-butyrolactone (II) sequentially into a 250mL four-necked bottle equipped with mechanical stirring, a condenser, and a thermometer, heat up to 100°C, stir and Add 85.0 g (1.0 mol) of butyrolactam (I) dropwise while keeping warm, keep warm for 2 hours, slowly pour into ice water when the temperature drops to 70°C, adjust to PH=2~3 with concentrated hydrochloric acid, filter, wash with water, and dry 103.1 g of white solid was obtained, the target compound N-(3-carboxypropyl)butyrolactam (III), with a melting point of 78-80° C., yield: 60.3%.
Embodiment 2
[0047] Preparation of N-(3-carboxypropyl)butyrolactam (Ⅲ)
[0048] Add 23.0 g (1.0 mol) of sodium metal and 86.0 g (1.0 mol) of γ-butyrolactone (II) sequentially into a 250 mL four-necked bottle equipped with mechanical stirring, a condenser, and a thermometer, raise the temperature to 130 ° C, and stir for 0.5 Hours, add 85.0g (1.0mol) of butyrolactam (I) dropwise while keeping warm, keep warm for 4 hours, slowly pour into ice water when the temperature is lowered to 70°C, adjust to PH=2~3 with concentrated hydrochloric acid, filter, wash with water, Drying gave 145.7g of white solid, melting point 78-80°C, which was the target compound N-(3-carboxypropyl)butyrolactam (Ⅲ), yield: 85.2%.
Embodiment 3
[0050] Preparation of N-(3-carboxypropyl)butyrolactam (Ⅲ)
[0051] Add 27.6g (1.2mol) of sodium metal and 86.0g (1.0mol) of γ-butyrolactone (II) sequentially into a 250mL four-necked bottle equipped with mechanical stirring, a condenser, and a thermometer, raise the temperature to 250°C, and stir for 1 Hours, add 85.0g (1.0mol) of butyrolactam (I) dropwise while keeping warm, keep warm for 8 hours, slowly pour into ice water when the temperature is lowered to 70°C, adjust to PH=2~3 with concentrated hydrochloric acid, filter, wash with water, Drying gave 150.0 g of a white solid with a melting point of 78-80° C., which was the target compound N-(3-carboxypropyl)butyrolactam (Ⅲ), yield: 87.7%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com