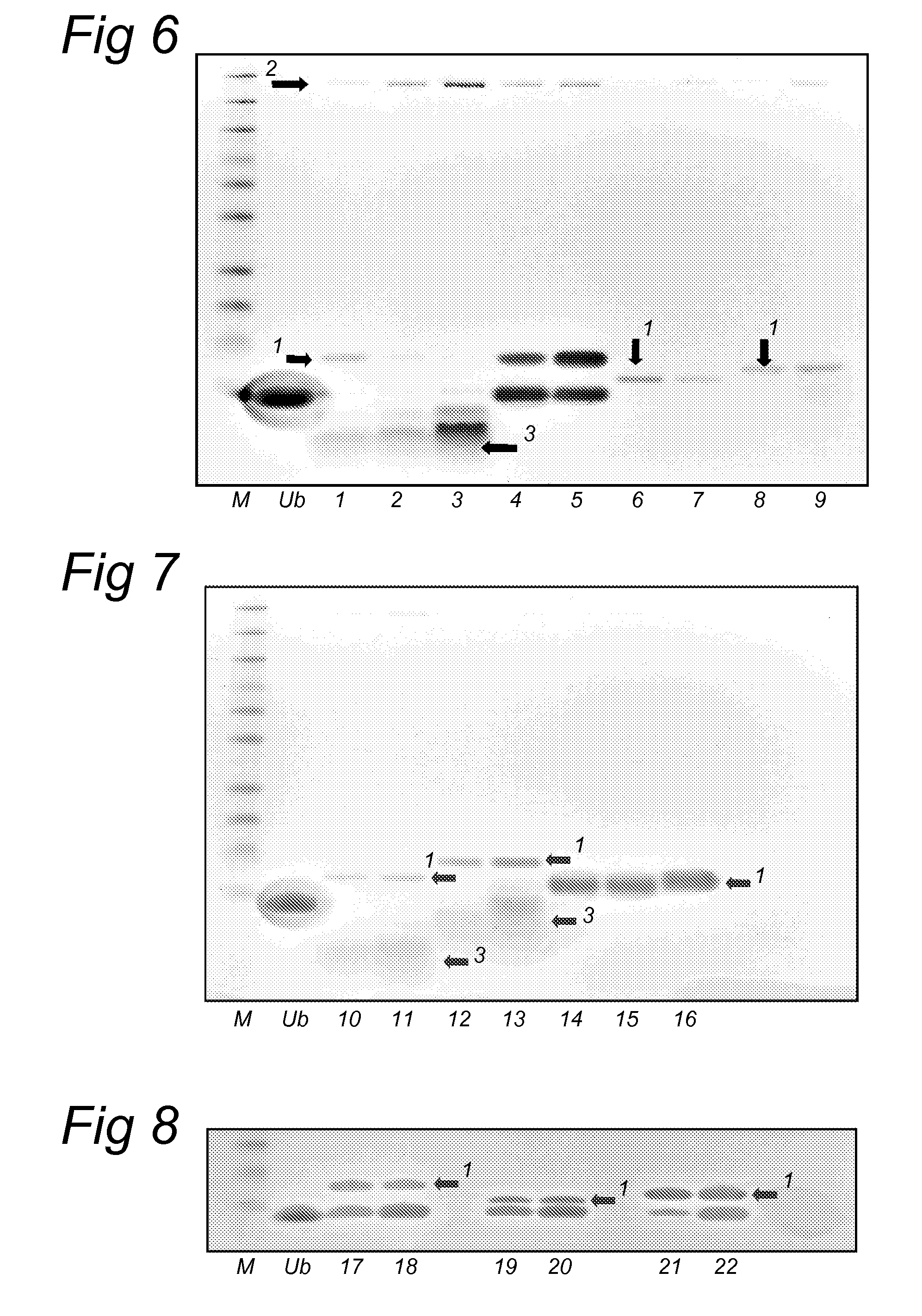
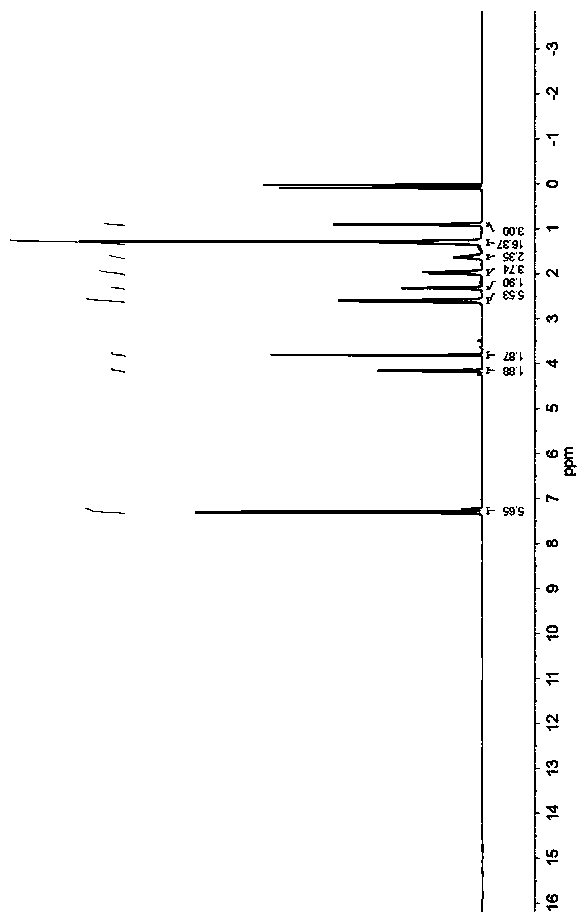
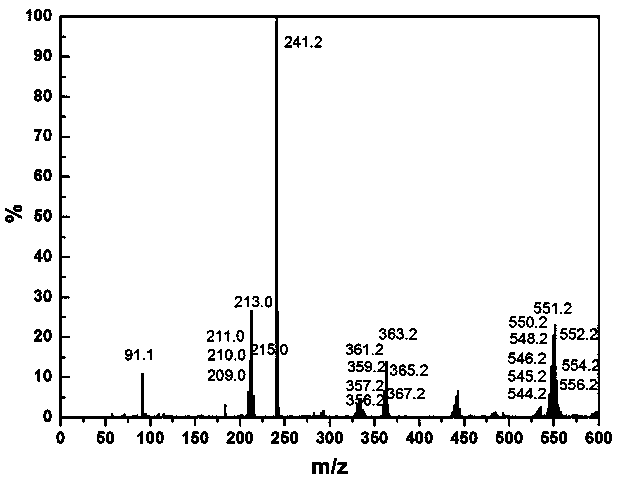
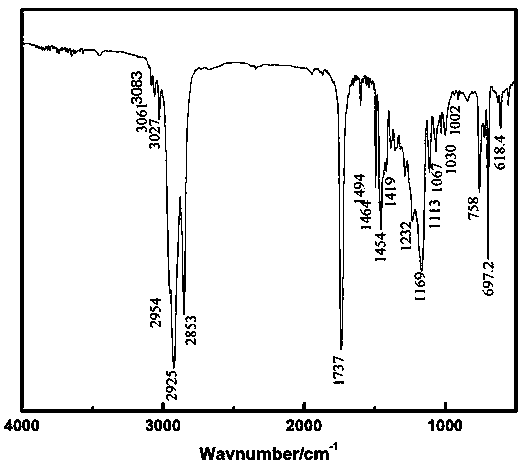
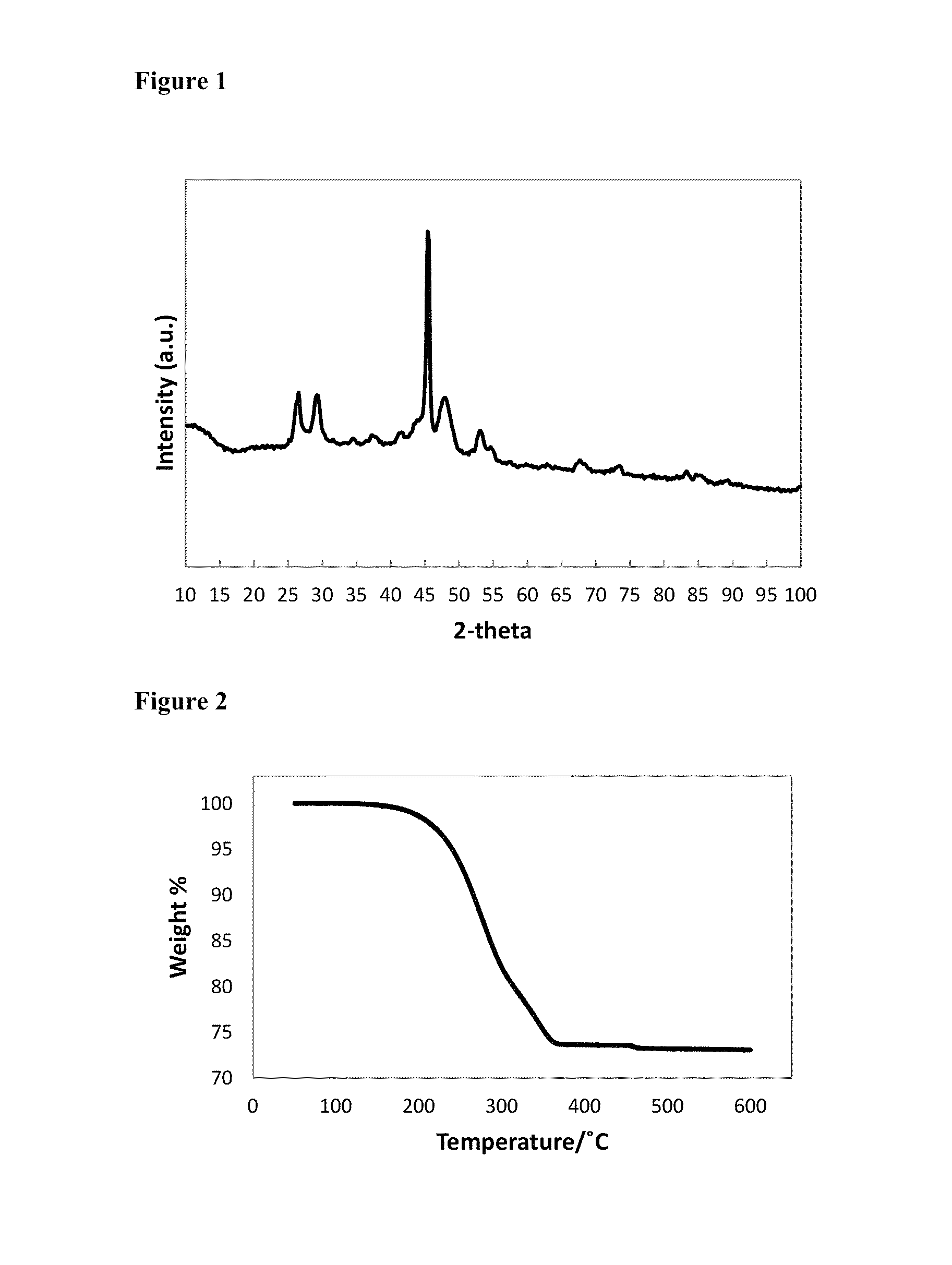
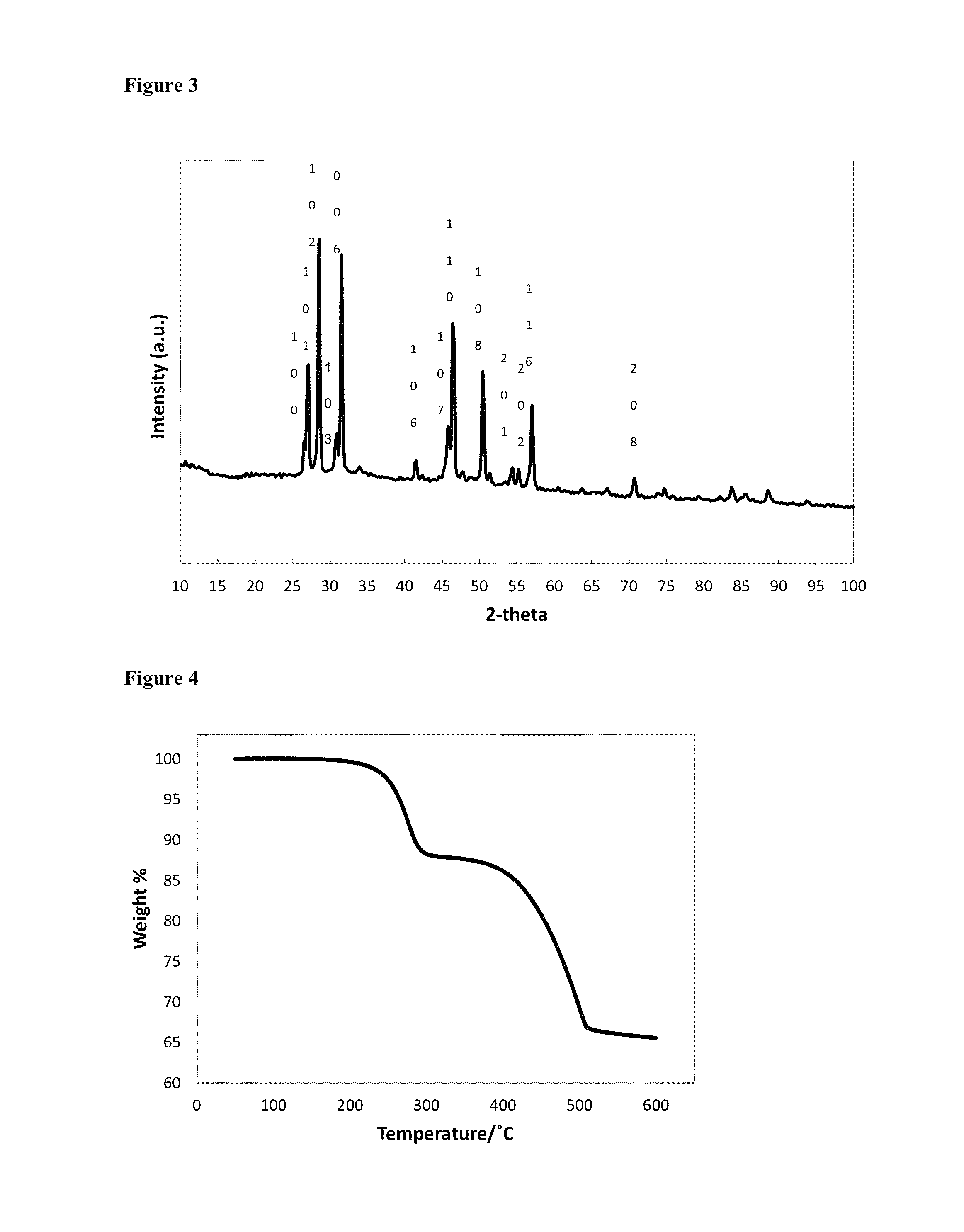
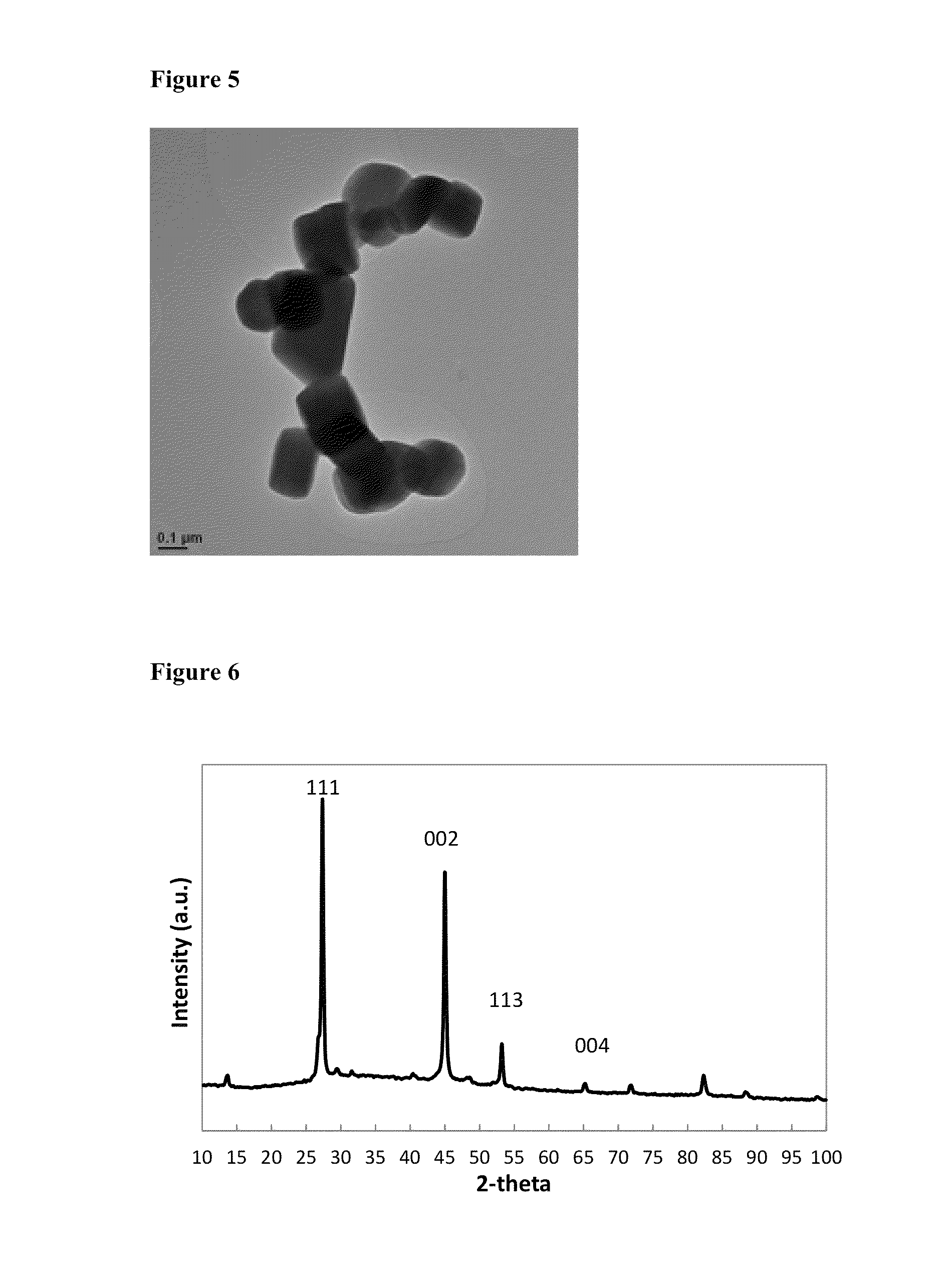
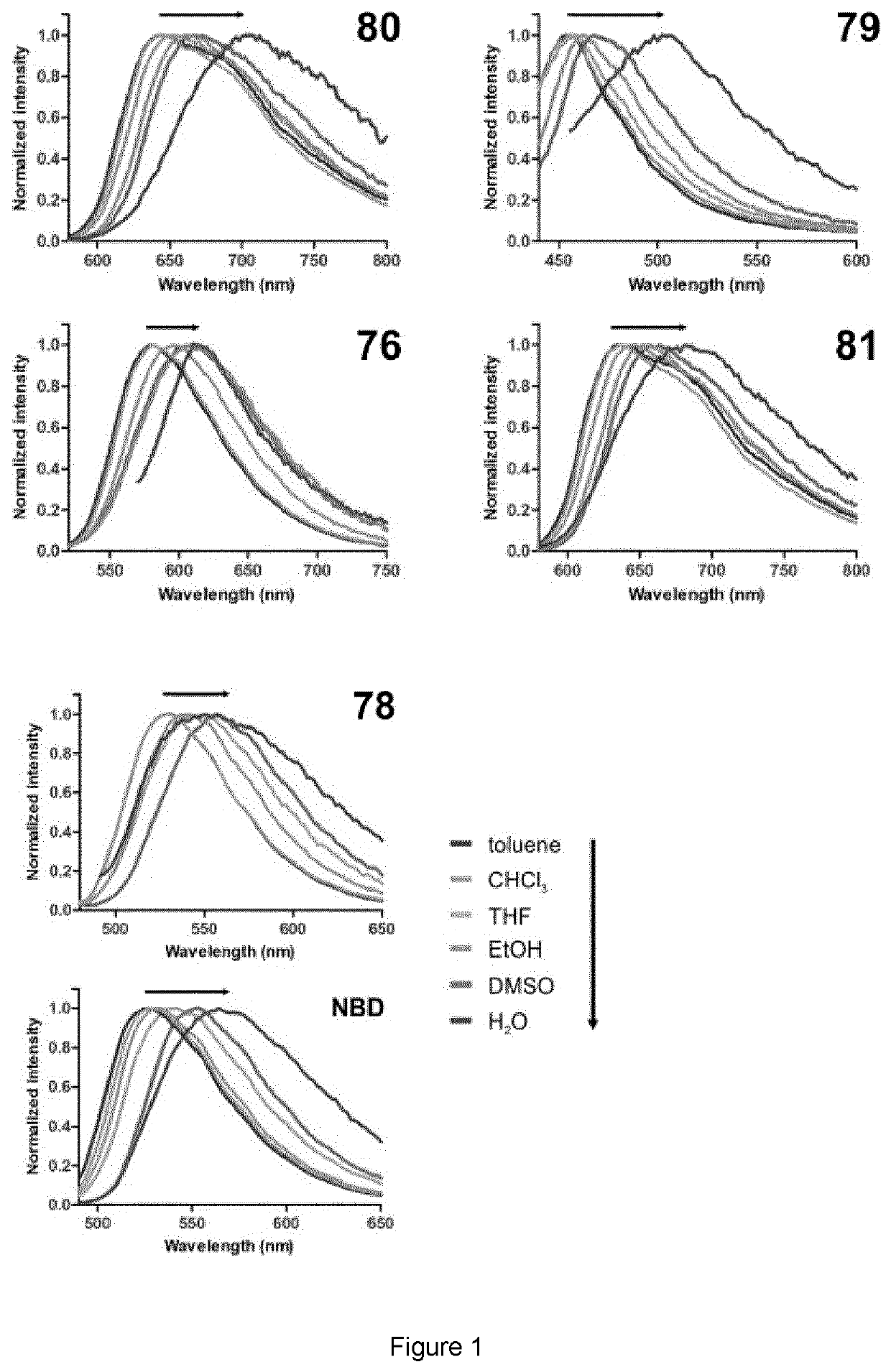
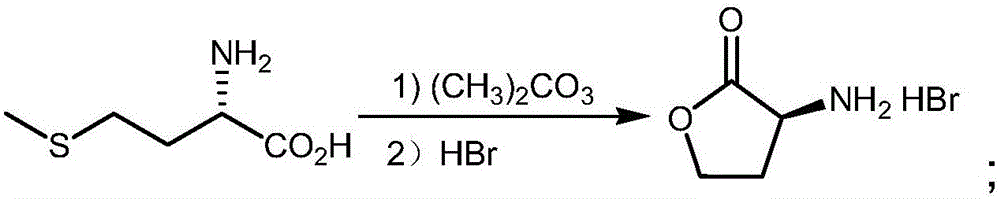Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
41 results about "Selenol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
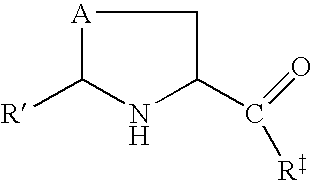
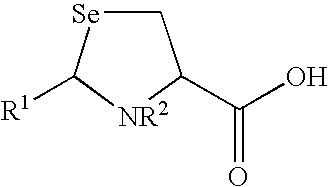
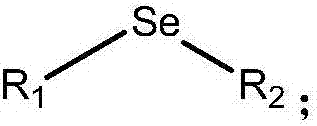
Selenols are organic compounds that contain the functional group with the connectivity C–Se–H. Selenols are sometimes also called selenomercaptans and selenothiols. Selenols are one of the principal classes of organoselenium compounds. The best known member is the amino acid selenocysteine.
Polymer nano hydrogel and preparation method thereof
ActiveCN102093555AImprove stabilityGood biocompatibilityPharmaceutical delivery mechanismPharmaceutical non-active ingredientsPolymer scienceDrug release
The invention provides a polymer nano hydrogel. A polymer matrix of the polymer nano hydrogel comprises a block copolymer with the structure shown in a formula (I) or a formula (II) in the specifications, and the block copolymer is subjected to internal crosslinking through Se-Se bond molecules. The invention further provides a preparation method of the polymer nano hydrogel. In the polymer nano hydrogel provided in the invention, the polymer matrix comprises a poly(L-glutamic acid) chain block containing carboxyl, the carboxyl is sensitive to a pH value and ion strength in a water solution, and the Se-Se bond is sensitive to both reductant and oxidant: in an oxidant environment, the Se-Se bond breaks to generate selenic acid and realize decrosslinking, and in an reductant environment, the Se-Se bond is reduced into selenol to realize decrosslinking. Therefore, the polymer nano hydrogel provided in the invention is simultaneously sensitive to the pH value, the ion strength, the oxidant and the reductant, and can realize quick drug release in target cells so as to improve the curative effect of the drugs.
Owner:CHANGZHOU INST OF ENERGY STORAGE MATERIALS &DEVICES
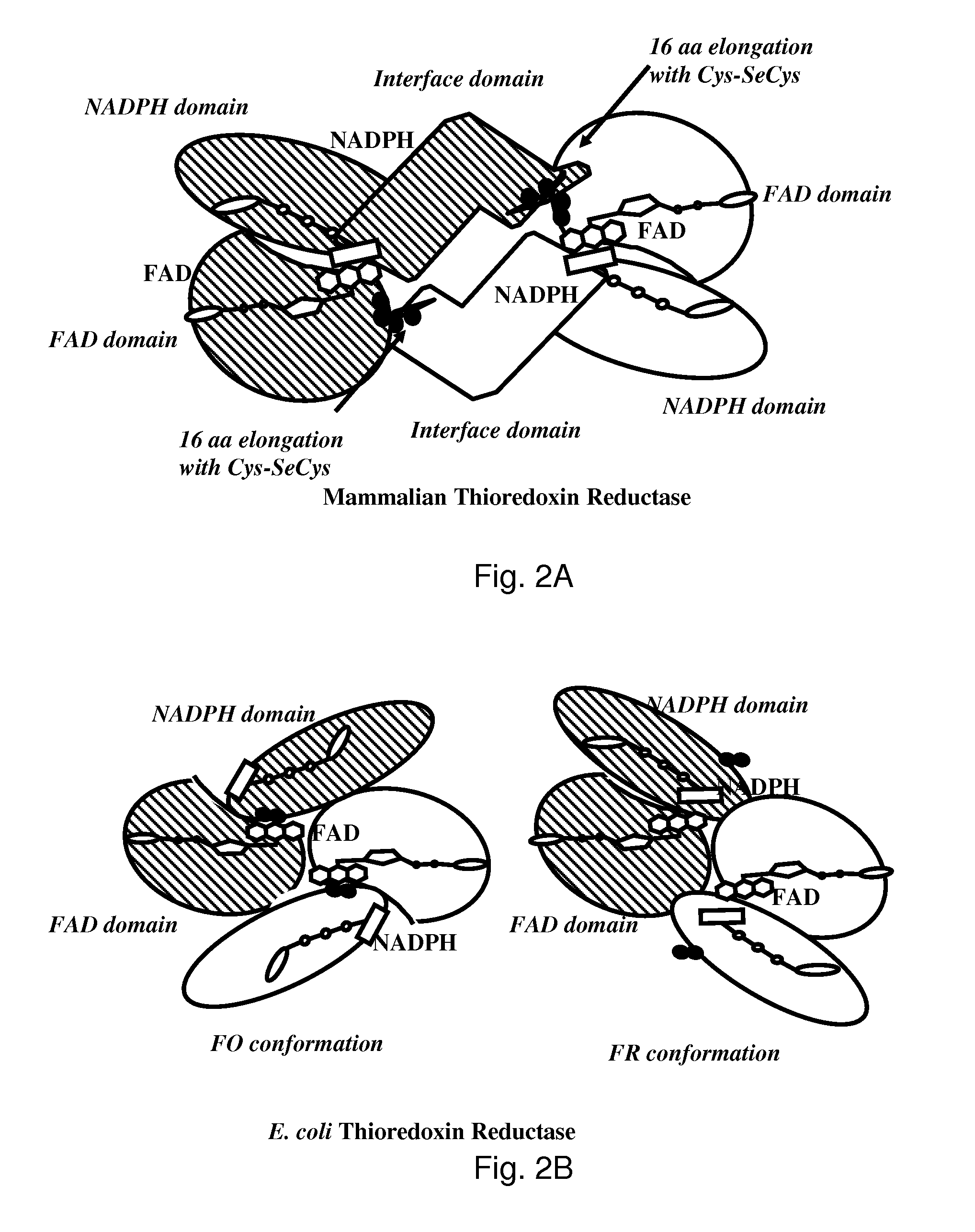
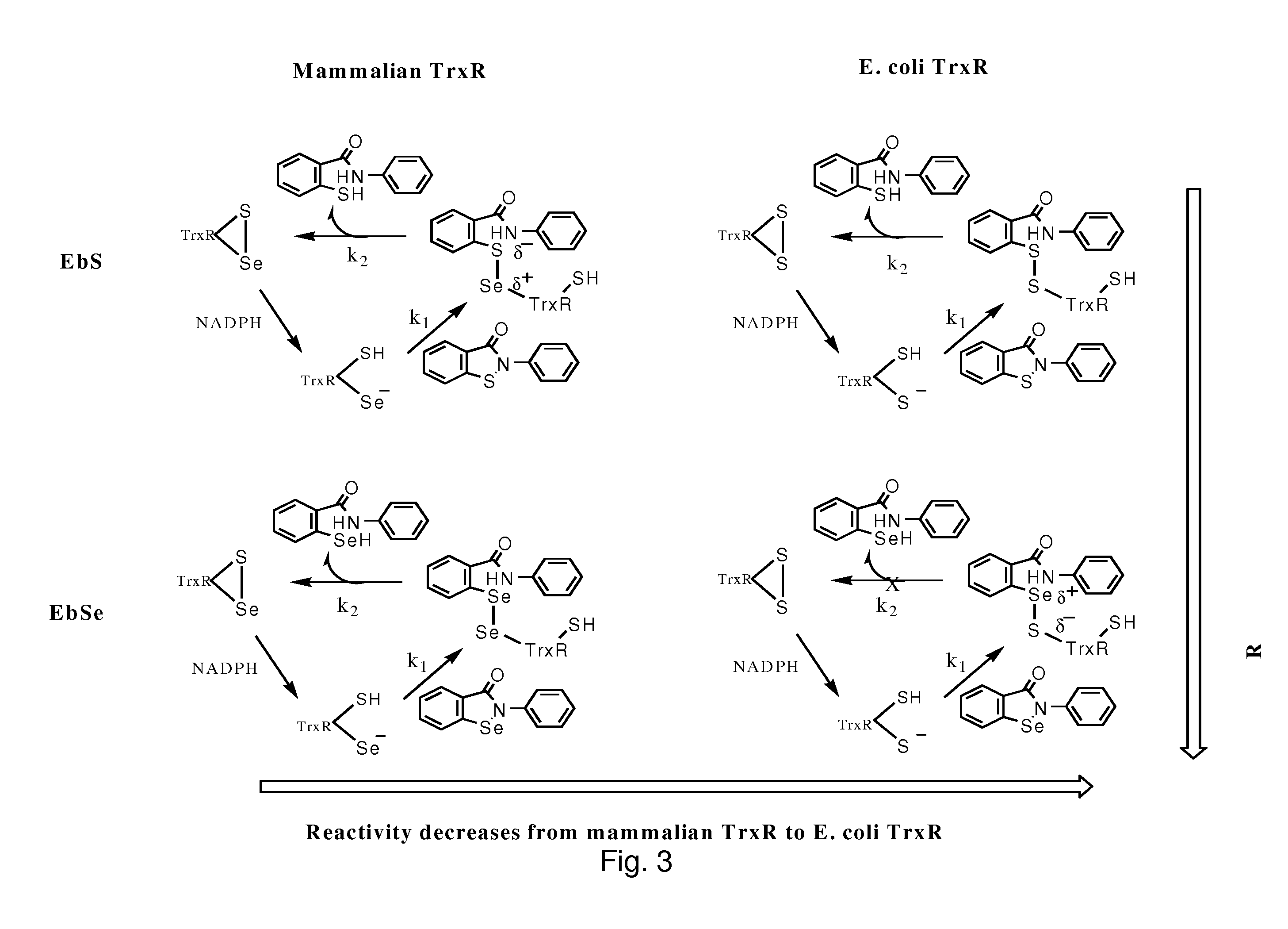
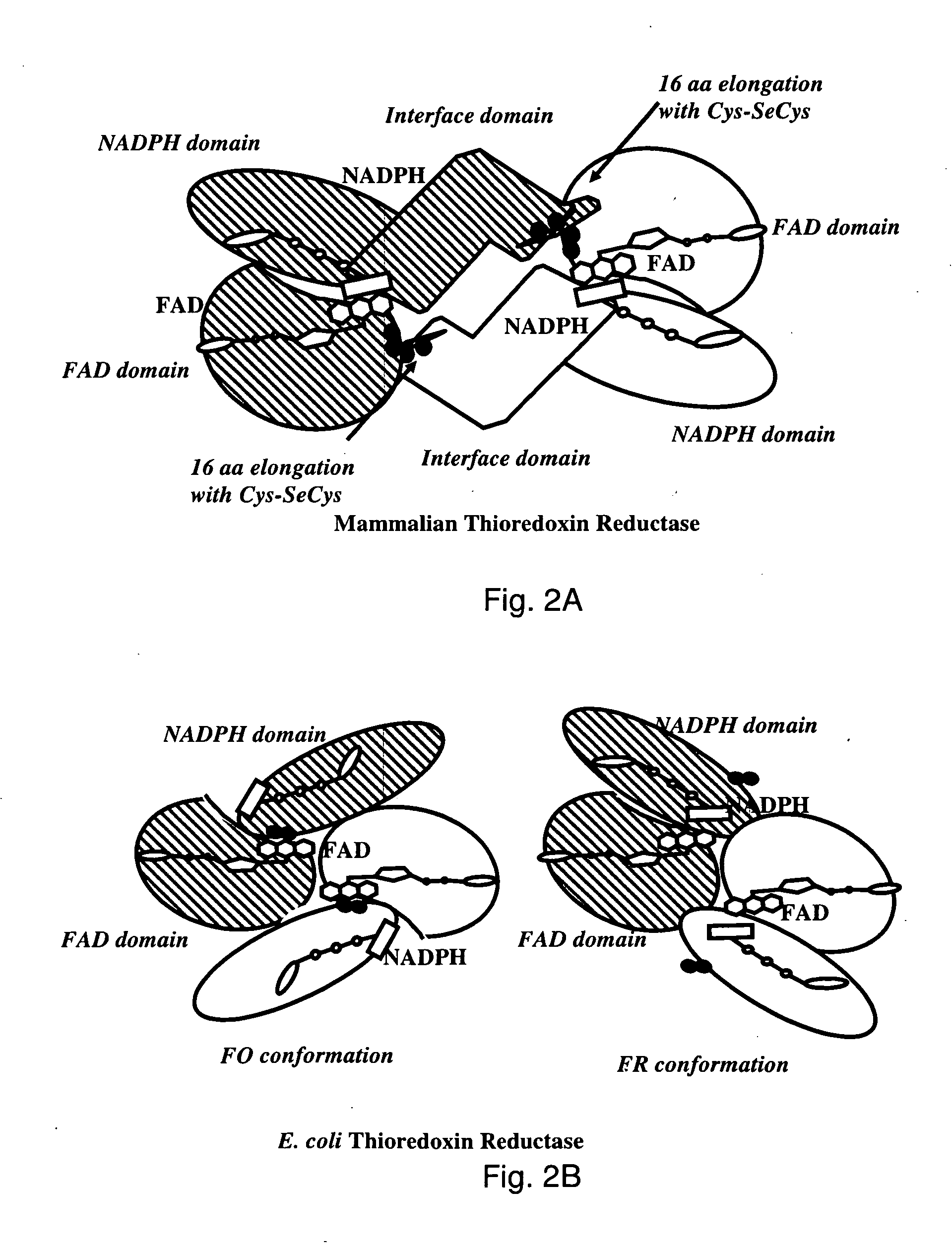
Bacterial thioredoxin reductase inhibitors and methods for use thereof
ActiveUS8592468B2Prevent bacterial growthImprove understandingAntibacterial agentsBiocideBacteroidesEscherichia coli
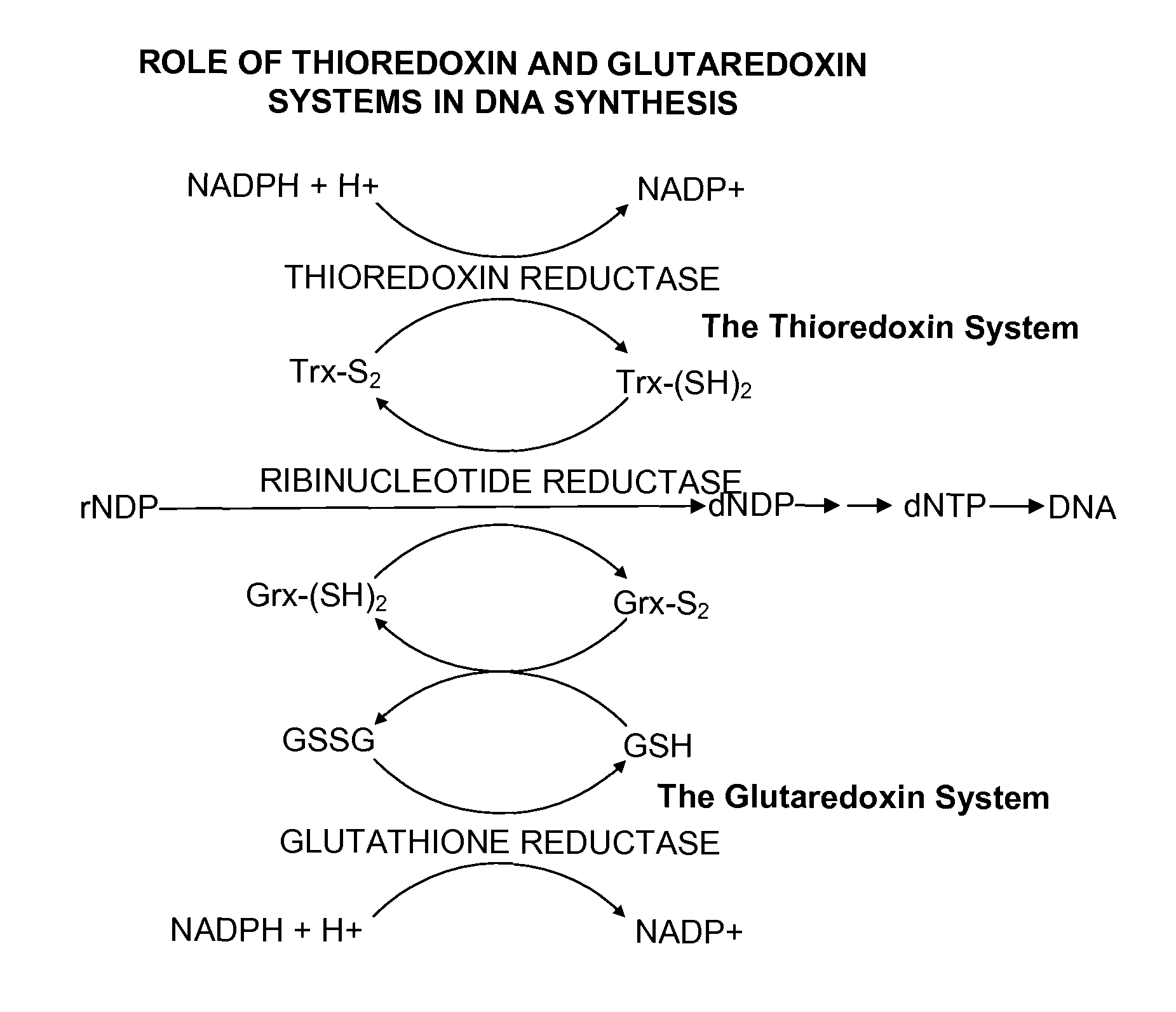
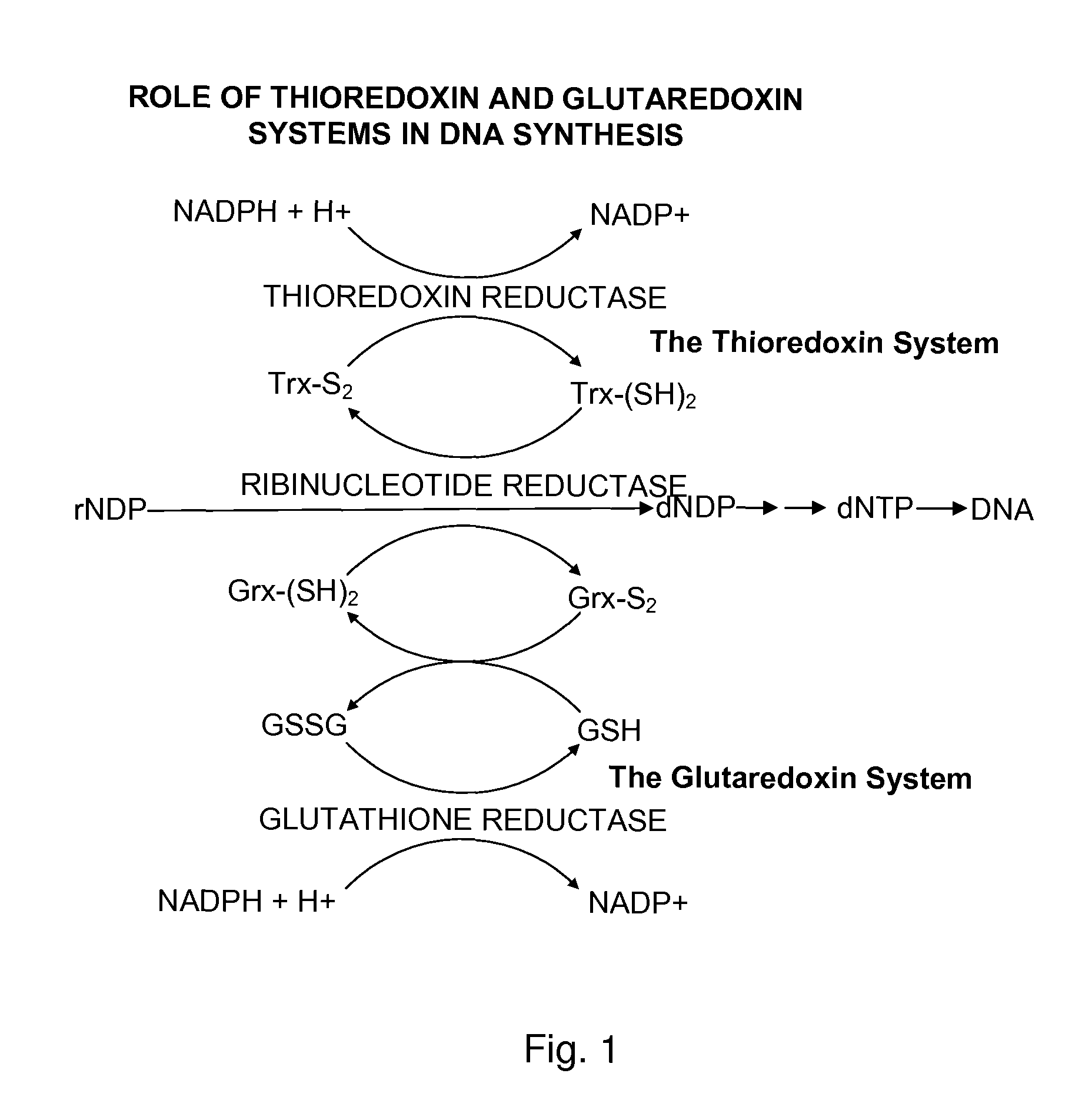
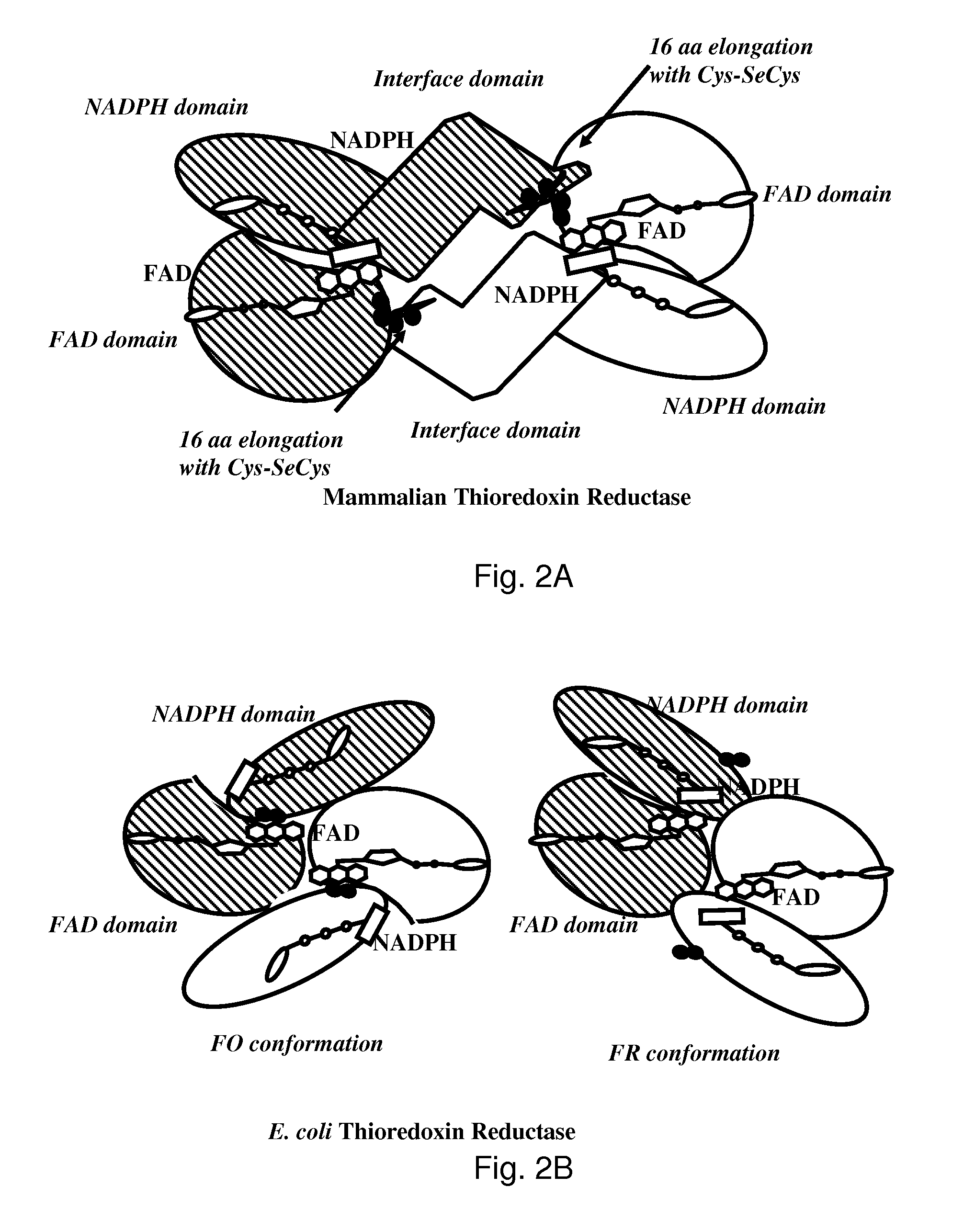
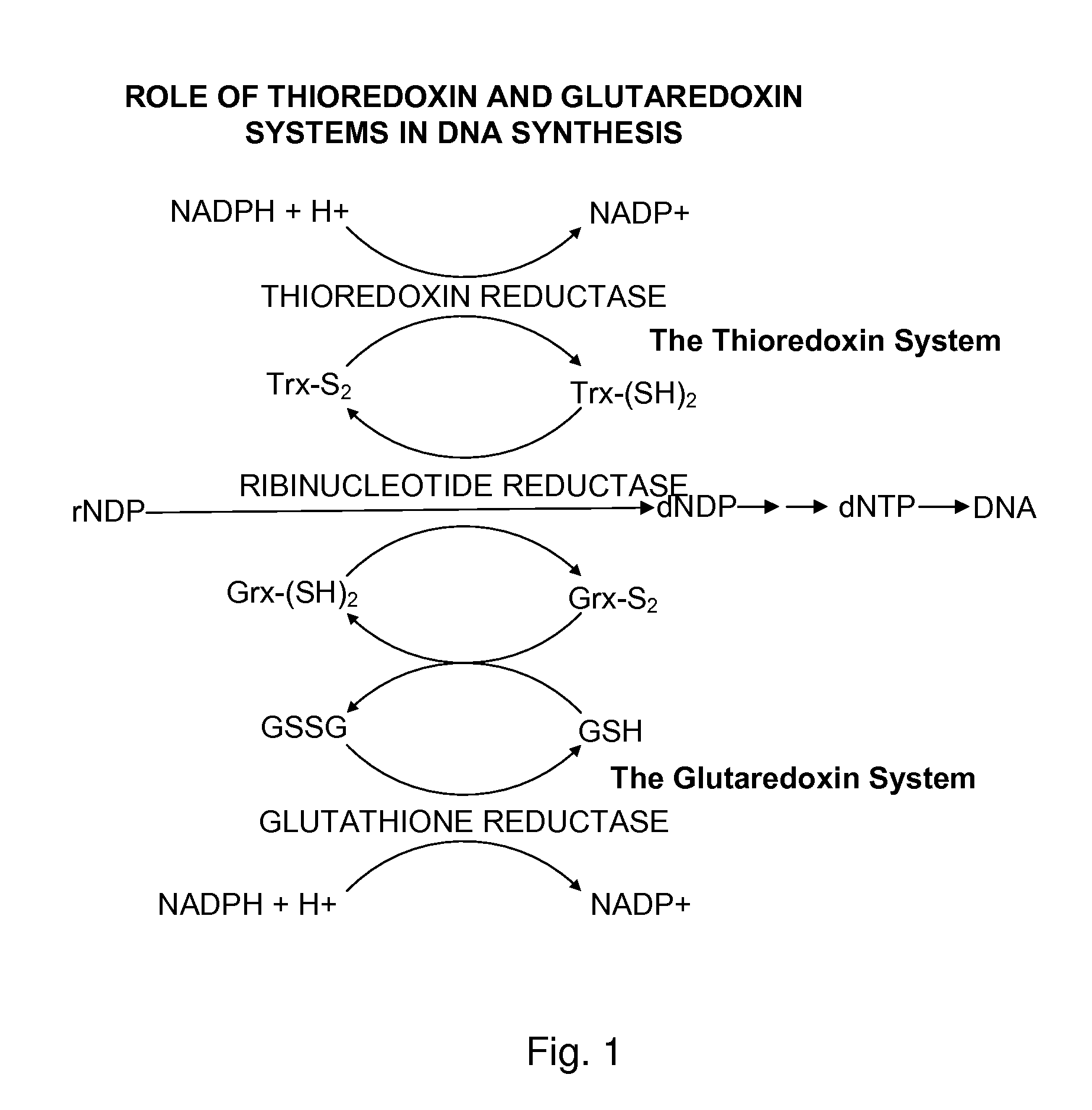
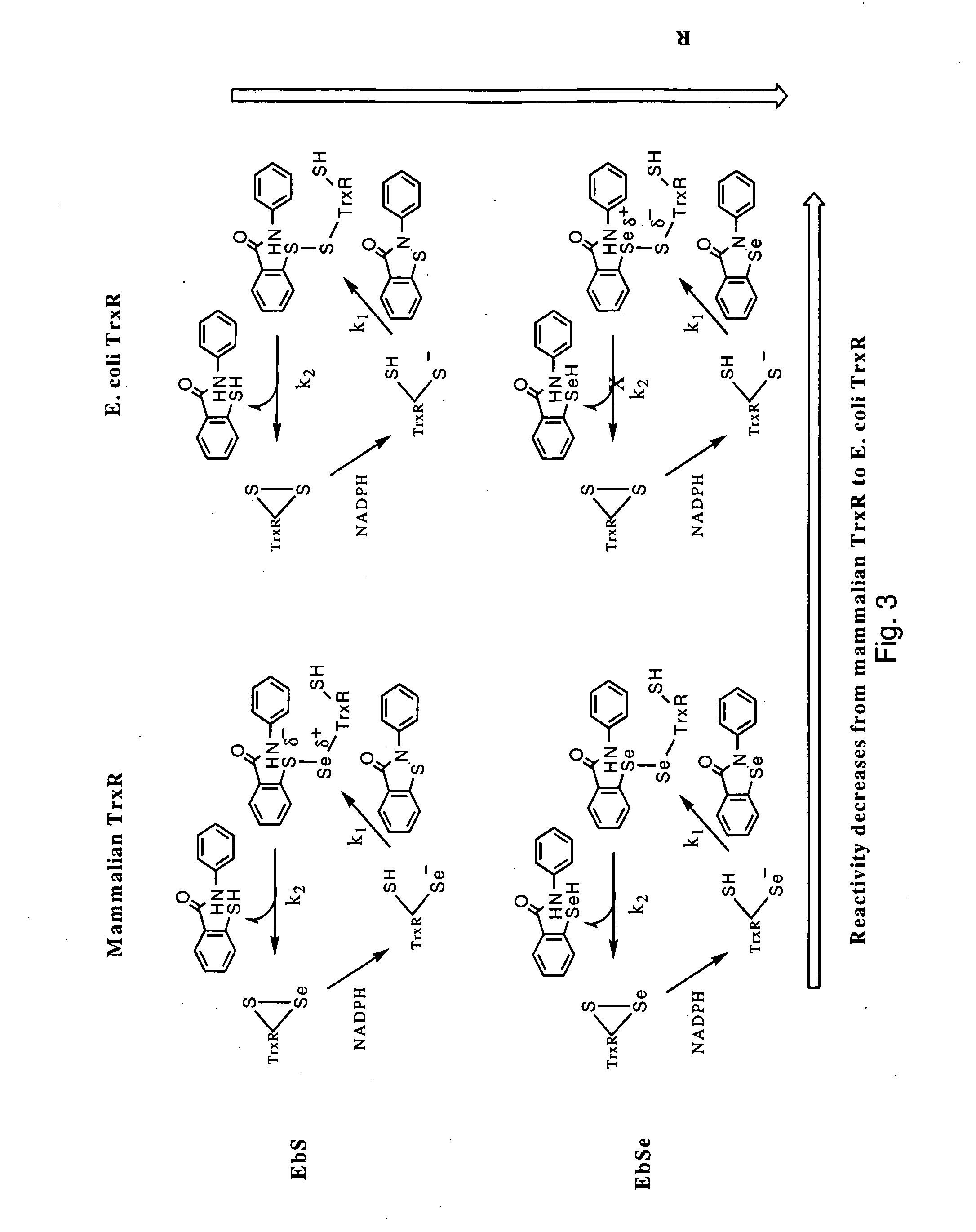
The mechanism of action of Ebselen differentiates between bacterial and mammalian thioredoxin reductase (TrxR). It displays fast oxidation of mammalian Trx and via the NADPH-TrxR catalyzed turnover of ebselen selenol with hydrogen peroxide, and therefore are mammalian antioxidants. Ebselen, and its diselenide, are strong competitive inhibitors of E. coli TrxR with Ki of 0.14 μM and 0.46 μM, respectively. E. coli mutants lacking glutathione reductase or glutathione were much more sensitive to inhibition by ebselen. Since either glutaredoxin or thioredoxin systems are electron donors to ribonucleotide reductase, ebselen targets primarily glutathione and glutaredoxin-negative bacteria, a class which includes major pathogens. Ebselen, and similar compounds are therefore useful as antibacterial agents, even for multiresistant strains. Two major pathogenic bacteria, which previously had not been known to be sensitive to ebselen, Mycobacterium tuberculosis (tuberculosis) and Helicobacter pylori (stomach ulcer and cancer), were shown to be excellent targets. Helicobacter pylori was also sensitive to ebsulfur.
Owner:THIOREDOXIN SYST AB
Compositions for the treatment and prevention of cancer
InactiveUS6867238B2BiocideSulfur/selenium/tellurium active ingredientsCancer preventionTransdermal patch
Compositions are provided for the topical treatment of cancer consisting of lotions, creams, sprays, suppositories or slow-release transdermal patches containing lipid-soluble, skin-penetrating organic selenium compounds in combination with inert carriers in therapeutically effective amounts of selenium compound. The selenium compounds are medium linear chain dialkyl diselenides and precursors such as alkyl selenols. Preferred compositions employ R—Se—Se—R compounds where R is from 6 to 8 carbon atoms, and most specifically di-n-hexyl diselenide. Commonly used carriers may be purified hydrocarbon fractions, oils, with or without added fat-soluble vitamins, water and emulsifying agents.
Owner:SCHRAUZER CAROL S
Prodrugs and conjugates of thiol- and selenol-containing compounds and methods of use thereof
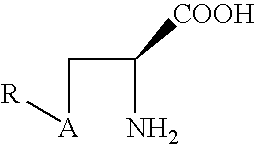
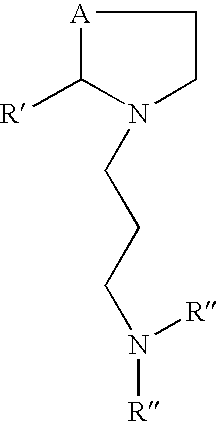
Disclosed is a prodrug of the formula: where A is a sulfur or a selenium, and R is derived from a mono- di- or oligo- saccharide.Also disclosed is a prodrug of the formula: where A is sulfur or selenium, R′ is derived from a sugar and R′ has the formula (CHOH)nCH2OH, where n is 1 to 5, or R′ is an alkyl or aryl group, orR′ is ═O, and the R″ groups may be the same or different and may be hydrogen, alkyl, alkoxy, carboxy.Also disclosed is a conjugate of an antioxidant vitamin and a thiolamine or selenolamine.Also disclosed is a prodrug of the formula; where A is sulfur or selenium, and R′ is derived from a sugar and R′ has the formula (CHOH)nCH2OH, where n is 1 to 5, or R′ is also be an alkyl or aryl group, orR′ is ═O, and R‡ is an alkoxy, or an amine group.Also disclosed is a prodrug of the formula: where R is COOH or H, and R′ is derived from a sugar and R′ has the formula (CHOH)nCH2OH,where n is 1 to 5, or R′ is an alkyl or aryl group, or R′ is ═O.
Owner:UNIV OF UTAH RES FOUND
Lysine compounds and their use in site- and chemoselective modification of peptides and proteins
The present invention concerns new thiolysine and selenolysine compounds that can be used as building blocks for peptides and proteins, providing ligation handles for site-and chemoselective modification of said peptides and proteins. In particular, the invention provides. In particular, the invention provides (the use of) the compounds 5-thiolysine (also referred to as d-thiolysine); 4-thiolysine (also referred to as ?-thiolysine); 5-selenolysine (also referred to as d-selenolysine) and 4- selenolysine (also referred to as ?-selenolysine).; The positioning of the thiol or selenol group at the respective carbon atom allows for a very efficient intramolecular transfer reaction to take place after conjugation with a selected ligand, and the thiol or selenol group may subsequently be removed using reported procedures, thereby restoring the native lysine structure, or be used as an additional conjugation handle. The methodology is fast and gives well-defined material.
Owner:NETHERLANDS CANCER INST FOUNDATION
Bacterial thioredoxin reductase inhibitors and methods for use thereof
ActiveUS20110288130A1Prevent bacterial growthImprove understandingAntibacterial agentsBiocideBacteroidesEscherichia coli
Owner:THIOREDOXIN SYST AB
Bacterial thioredoxin reductase inhibitors and methods for use thereof
InactiveUS20090005422A1Reduced thioredoxinPrevent bacterial growthAntibacterial agentsBiocideBacteroidesEscherichia coli
The mechanism of action of Ebselen differentiates between bacterial and mammalian thioredoxin reductase (TrxR). It displays fast oxidation of mammalian Trx and via the NADPH-TrxR catalyzed turnover of ebselen selenol with hydrogen peroxide, and therefore are mammalian antioxidants. Ebselen, and its diselenide, are strong competitive inhibitors of E. coli TrxR with Ki of 0.14 μM and 0.46 μM, respectively. E. coli mutants lacking glutathione reductase or glutathione were much more sensitive to inhibition by ebselen. Since either glutaredoxin or thioredoxin systems are electron donors to ribonucleotide reductase, ebselen targets primarily glutathione and glutaredoxin-negative bacteria, a class which includes major pathogens. Ebselen, and similar compounds are therefore useful as antibacterial agents, even for multiresistant strains. Two major pathogenic bacteria, which previously had not been known to be sensitive to ebselen, Mycobacterium tuberculosis (tuberculosis) and Helicobacter pylori (stomach ulcer and cancer), were shown to be excellent targets. Helicobacter pylori was also sensitive to ebsulfur.
Owner:THIOREDOXIN SYST AB
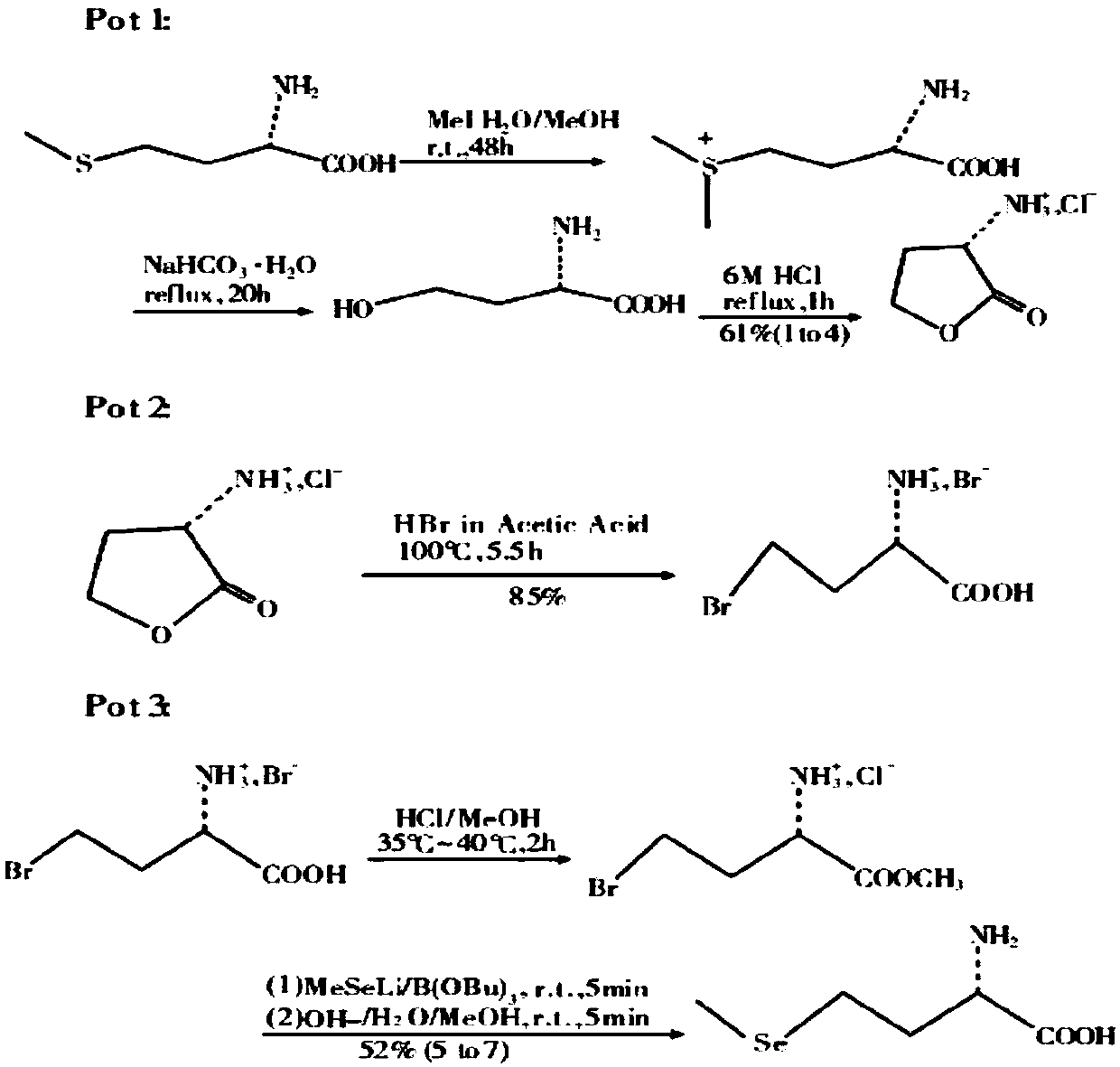
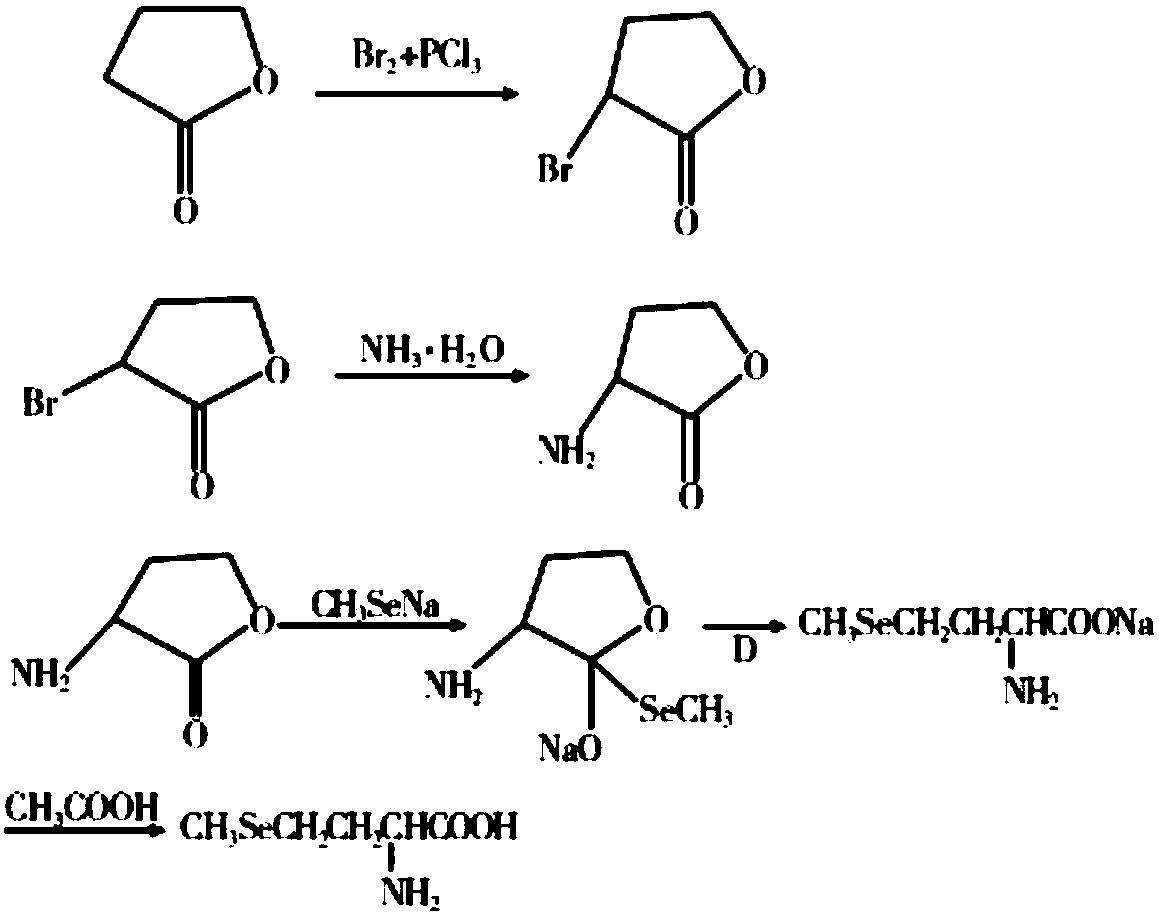
Preparation method for selenomethionine
ActiveCN106220539AThe synthesis process is simpleMild reaction conditionsOrganic chemistrySodium saltDiselenide
The invention discloses a preparation method for selenomethionine, which includes the steps of: (a) performing a reaction to L-methionine and dimethyl carbonate to remove a methylthio group and meanwhile form a ring to generate L-[alpha]-amino-[gamma]-butyrolactone, and adding hydrobromic acid to carry out a reaction to obtain L-[alpha]-amino-[gamma]-butyrolactone hydrobromate; (b) performing a reaction to selenium with hydrazine hydrate to generate sodium diselenide, adding a methylation reagent, dimethyl carbonate, to carry out a reaction to generate dimethyl diselenide, and reducing the dimethyl diselenide with sodium borohydride to obtain sodium methylselenide; and (c) performing a heating reflux reaction to the L-[alpha]-amino-[gamma]-butyrolactone hydrobromate and sodium methyl selenol to obtain L-selenomethionine sodium salt, and regulating the pH of the reaction liquid to 5-6 with acetic acid, and dehydrating the reaction liquid to obtain the target product L-selenomethionine. The preparation method has high product yield and simple synthetic process, is prepared from easy-to-obtain raw materials, has mild reaction conditions and is easy to carry out industrially.
Owner:SICHUAN SINYIML BIOTECH CO LTD
Prodrugs and conjugates of thiol- and selenol-containing compounds and methods of use thereof
Disclosed are compounds having the formula wherein R1 is hydrogen, an alkyl group, an aryl group, a cycloalkyl group, an alkenyl group, an alkynyl group, an aralkyl group, or ═O, R2 is an alkyl group, an aryl group, a cycloalkyl group, an alkenyl group, an alkynyl group, or an aralkyl group, or the pharmaceutically acceptable salt or ester thereof. Also disclosed are methods of using the compounds.
Owner:UNIV OF UTAH RES FOUND
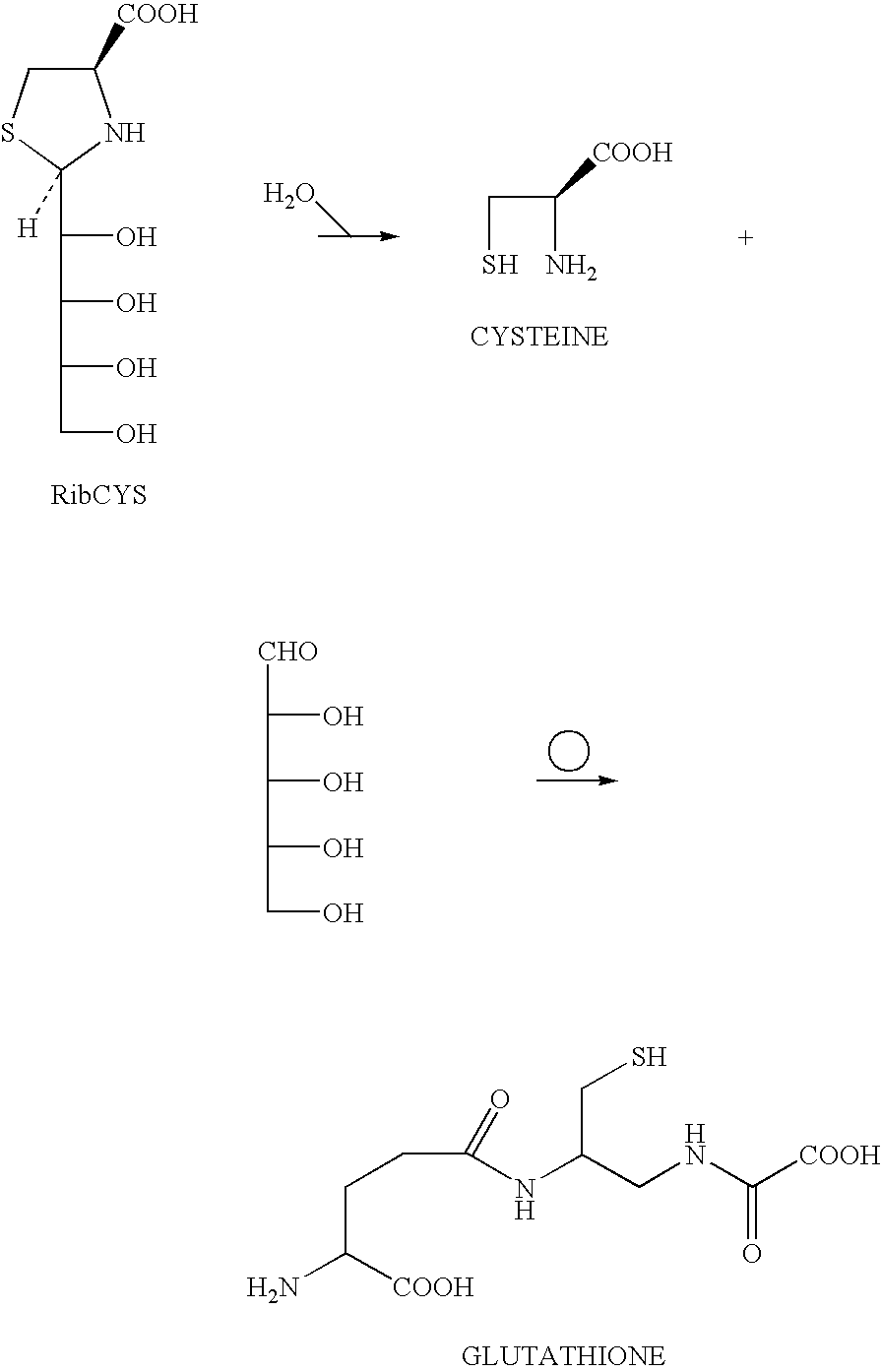
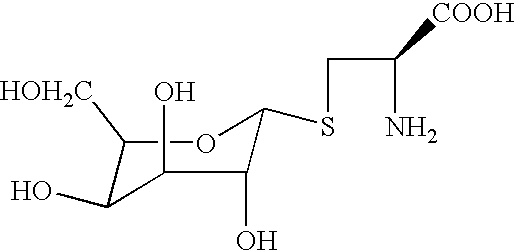
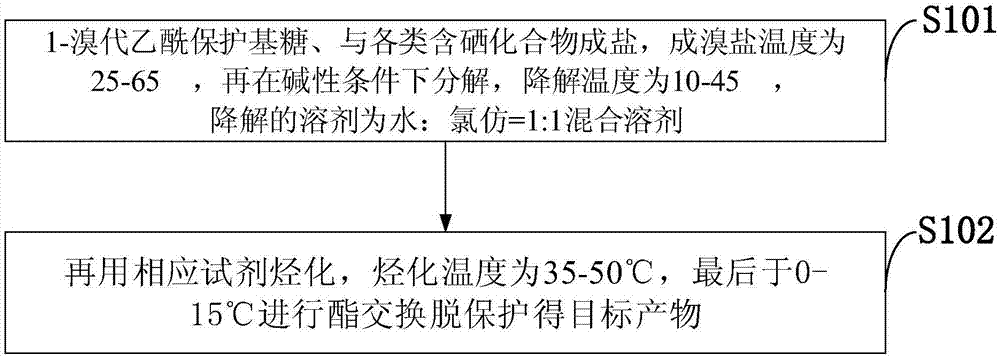
Selenium-containing compound selenium sugar, selenium glucoside and preparation method thereof
InactiveCN107344953ASimple structureConducive to in-depth developmentSugar derivativesSugar derivatives preparationPotassiumSodium selenide
The invention belongs to the technical field of selenium-containing compounds and discloses a selenium-containing compound selenium sugar, selenium glucoside and a preparation method thereof. The method comprises the following steps: using corresponding glycosyl bromide protected by a protecting group for reacting with selenourea and then degrading under an alkaline condition, or using the corresponding glycosyl bromide protected by the protecting group for directly reacting with selenium hydrogen silicate or using the corresponding glycosyl bromide protected by the protecting group for reacting with sodium selenide and potassium selenide; using the acquired raw material for reacting with a corresponding hydrocarbylation reagent or the corresponding glycosyl bromide protected by the protecting group represented by R2 or using the corresponding glycosyl bromide protected by the protecting group for directly reacting the corresponding selenol or sodium selenium alcohol and selenium potassium alcohol; and lastly, removing protection, thereby acquiring target compounds. The compound prepared according to the invention can be used for obviously improving the absorption and conversion of organism for selenium element, so that the selenium element can be utilized by a human body.
Owner:CHENGDU LAURELSCI TECH +1
Preparation of Copper Selenide Nanoparticles
ActiveUS20150024543A1Uniform sizeNarrow melting point rangeConductive materialSolid-state devicesNanoparticleSelenol
A process for producing copper selenide nanoparticles by effecting conversion of a nanoparticle precursor composition comprising copper and selenide ions to the material of the copper selenide nanoparticles in the presence of a selenol compound. Copper selenide-containing films and CIGS semiconductor films produced using copper selenide as a fluxing agent are also disclosed.
Owner:NANOCO TECH LTD
Two-dimension transition metal sulphur group compound and preparation method and device thereof
PendingCN111893456AUniform nucleationUniform growthChemical vapor deposition coatingSelenolLiquid state
The invention discloses a two-dimension transition metal sulphur group compound and a preparation method and device thereof. The preparation method comprises the following steps that a liquid state transition metal source is taken, and a substrate is covered by the liquid state transition metal source; a carrier gas is utilized to convey a liquid state sulphur group source to the position of the substrate, wherein the sulphur group source is mercaptan, selenol or telluromercaptan; and heating reaction is conducted in an enclosed environment, and the two-dimension transition metal sulphur groupcompound is acquired. According to the preparation method for the two-dimension transition metal sulphur group compound, the liquid state transition metal source and liquid state sulphur group sourceare utilized and serve as reaction precursors, it is easy to construct a stable precursor concentration field growth system through control over the concentration of the reaction precursors; thus uniform nucleation and growth of the transition metal sulphur group compound is promoted so that density distribution of the morphology, thickness and crystal domain dimension of the transition metal sulphur group compound material acquired through preparation on the substrate is more uniform; and the preparation method has outstanding optical and electrical properties and a broad application prospect.
Owner:TSINGHUA BERKELEY SHENZHEN INST
Surface display of selenocysteine-containing peptides
InactiveUS20050048548A1High binding activityMicrobiological testing/measurementMicroorganism librariesSurface displayRandom Peptide Library
The naturally-occurring amino acid selenocysteine (Sec) is incorporated uniquely and specifically in the context of a polypeptide displayed on the surface of an amplifiable genetic particle (phage, cell or spore) in response to incorporation signals engineered in the encoding DNA. In addition to conferring the unique activities of the selenol group to the chemistry of the displayed peptide, Sec also provides a unique handle for specific chemical modification of the displayed peptide. In addition to increasing the palette of available residues in a random peptide library to 21 possibilities, the present invention also provides a means of tethering virtually any desired chemical functionality to the incorporated Sec.
Owner:NEW ENGLAND BIOLABS
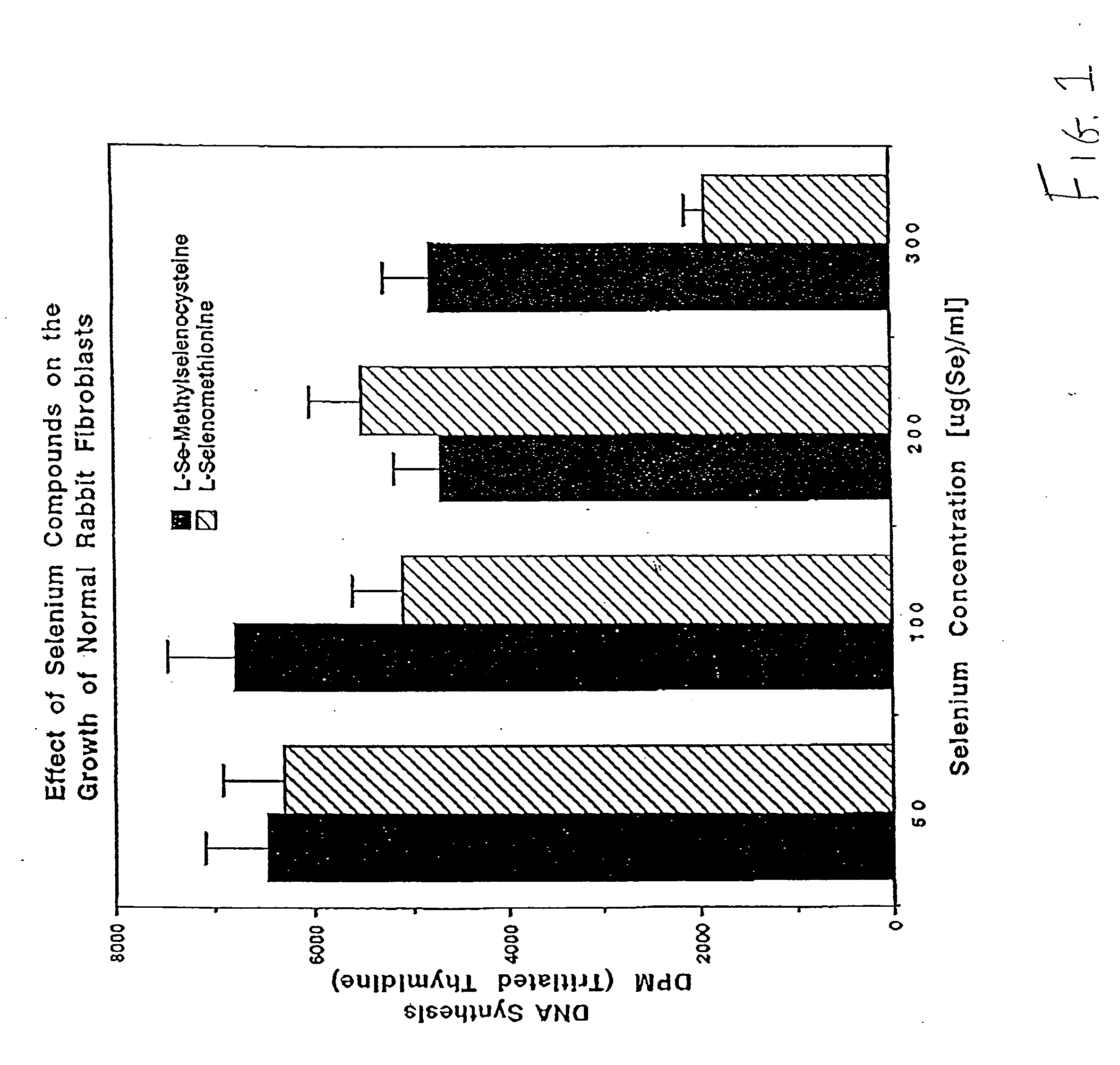
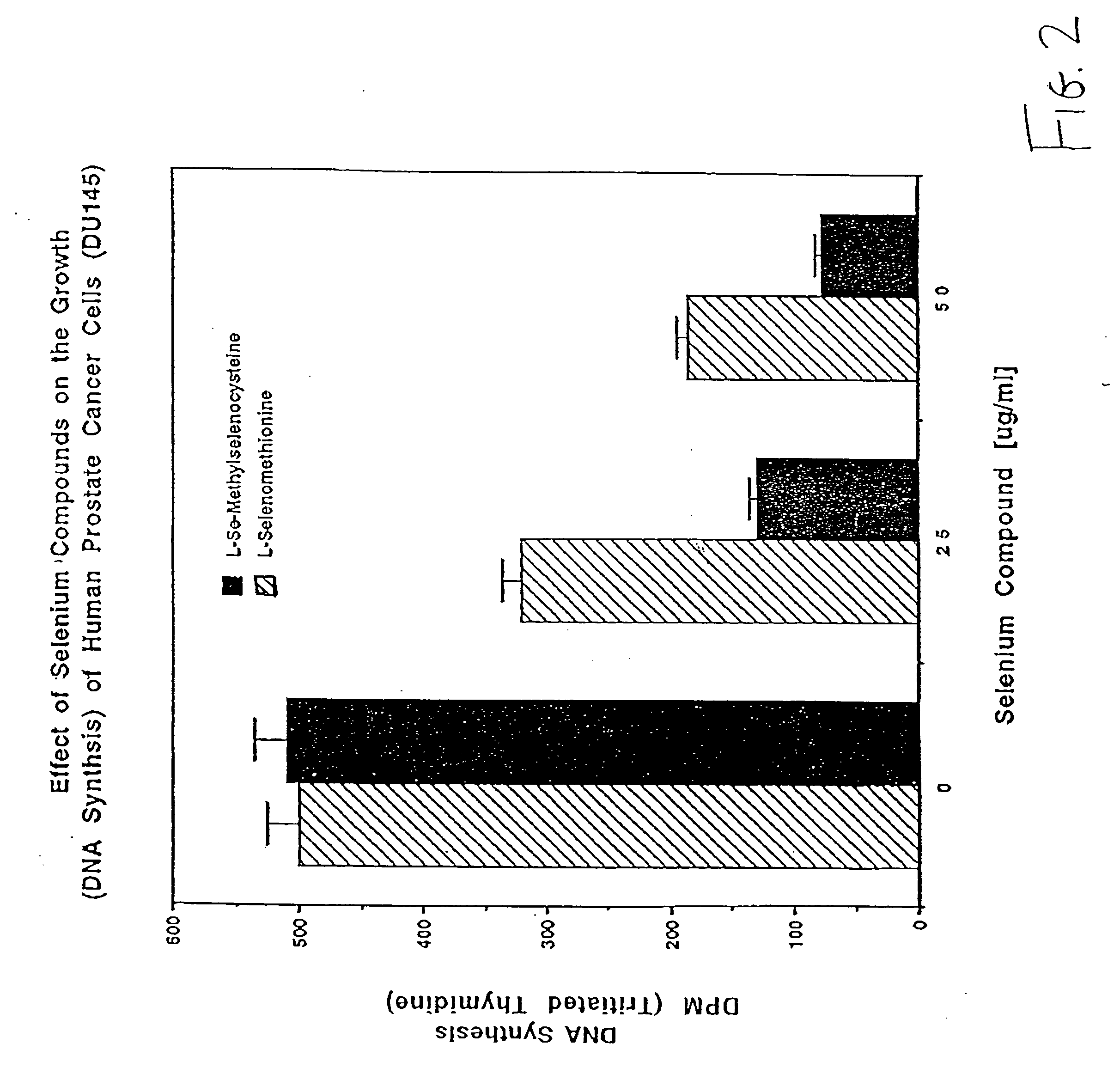
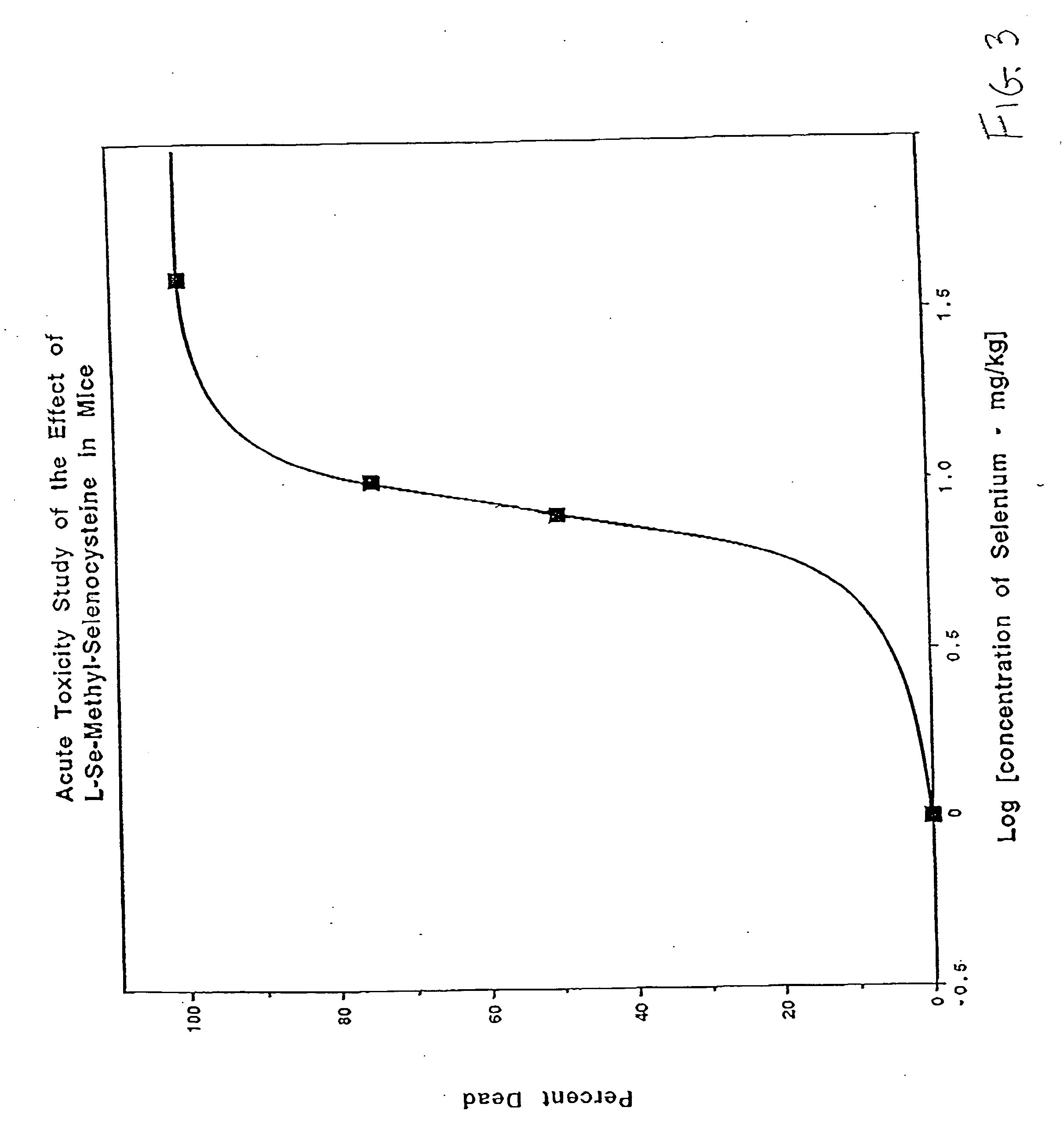
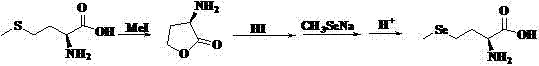
Method of using synthetic L-Se-methylselenocysteine as a nutriceutical and a method of its synthesis
InactiveUS20070088086A1Prevent and reduce riskToxic reductionOrganic active ingredientsBiocideSynthesis methodsTert-Butyloxycarbonyl protecting group
A synthesis of and use for L-Se-methylselenocysteine as a nutriceutical is described, based upon the knowledge that L-Se-methylselenocysteine is less toxic than L-selenomethionine towards normal cells. The synthesis proceeds by mixing N-(tert-butoxycarbonyl)-L-serine with a dialkyl diazodicarboxylate and at least one of a trialkylphosphine, triarylphosphine, and phosphite to form a first mixture that includes N-(tert-butoxycarbonyl)-L-serine β-lactone. Methyl selenol or its salt is mixed with the N-(tert-butoxycarbonyl)-L-serine β-lactone to form a second mixture that includes N-(tert-butoxycarbonyl)-Se-methylselenocysteine. The tert-butoxycarbonyl group is removed from the N-(tert-butoxycarbonyl)-Se-methylselenocysteine to form L-Se-methylselenocysteine. This synthesis significantly improves the manufacturability, manufacturing efficiency, and utility of this naturally occurring rare form of organic-selenium. L-Se-methylselenocysteine formed, for example, in this manner may be used as a nutriceutical for supplementation into the diets of humans or animals for various beneficial purposes, such as, for example, to prevent or reduce the risk of developing cancer.
Owner:PHARMASE
BODIPY derivative for measuring CysSnSSH and application thereof
ActiveCN105001250AReduce processing timeReduce testing costsGroup 3/13 element organic compoundsFluorescence/phosphorescencePhenacylBiotin
The invention relates to a fluorescent probe for detecting CysSnSSH (n is larger than one), in particular to a BODIPY derivative for measuring the CysSnSSH and application thereof. The BODIPY derivative is shown in the general formula I. In the general formula I, R1 (a detection group) is a sulfydryl benzoyl group or an aryl selenol benzoyl group or a tellurium phenol benzoyl group; R2 (a positioning functional group) is a sulfydryl benzoyl group, an aryl selenol benzoyl group, a tellurium phenol benzoyl group, a butyl triphenyl phosphonium group, an N-propyl morpholino group, a biotin group, a folic acid group, a glycosyl chain group or a C1-25 alkyl group; X represents H or halogen; Y represents N or C; Z represents N or O. In the presence of CysSnSSH, corresponding fluorescence intensity changes, and the compound like the CysSnSSH fluorescent probe can be used for detecting CysSnSSH, interference of external detection conditions can be greatly reduced, the sample treatment time is saved, and the detection precision is improved.
Owner:YANTAI INST OF COASTAL ZONE RES CHINESE ACAD OF SCI
Method for producing selenol
The main purpose of the present invention is to provide a method for producing a selenol with high yield without causing the fluctuations in yield among production lots. The purpose can be achieved by a method for producing a selenol, which comprises steps (1) and (2): (1) a step of reacting a Grignard reagent represented by general formula (1) with selenium to produce a reaction solution containing a selenomagnesium halide represented by general formula (2); and (2) a step of adding the reaction solution produced in step (1) mentioned above to an acidic solution dropwisely to produce a selenol represented by general formula (3).
Owner:SUMITOMO SEIKA CHEM CO LTD
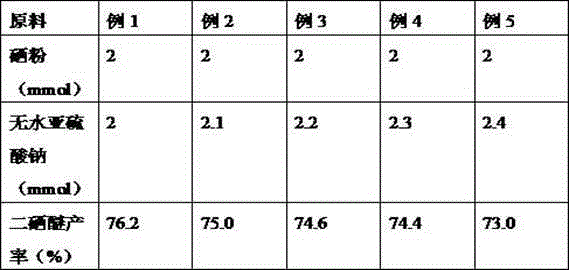
Diselenide synthesizing method
ActiveCN105130864ALess corrosiveSuitable for industrial productionOrganic chemistryChemical synthesisSulfite salt
Owner:四川硒莱坞科技有限公司
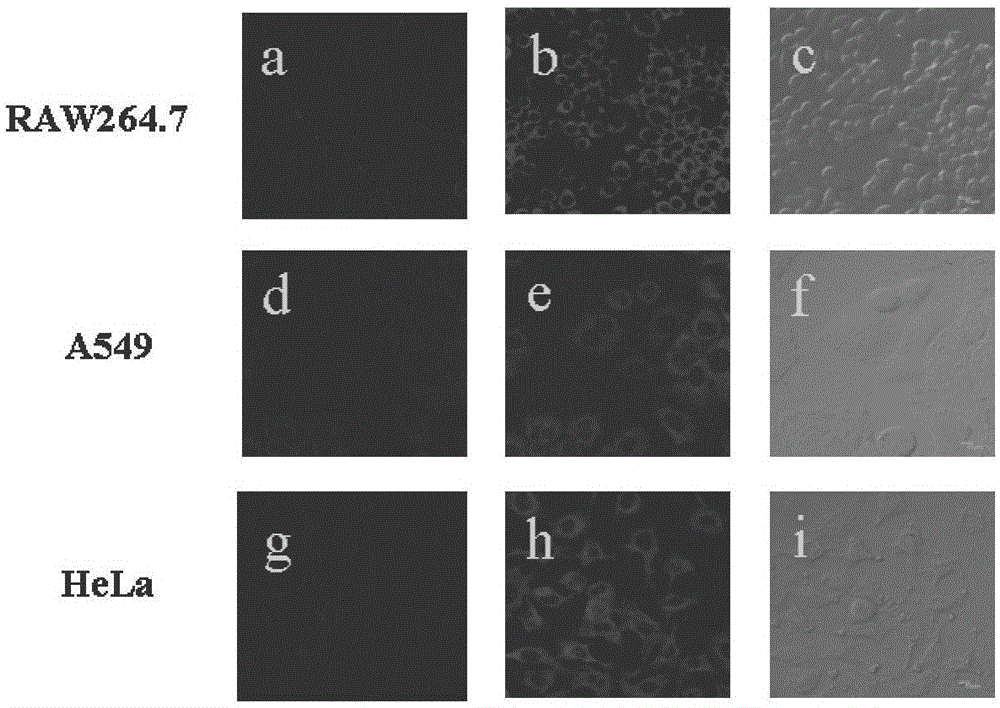
New method for detecting selenocysteine in living bodies
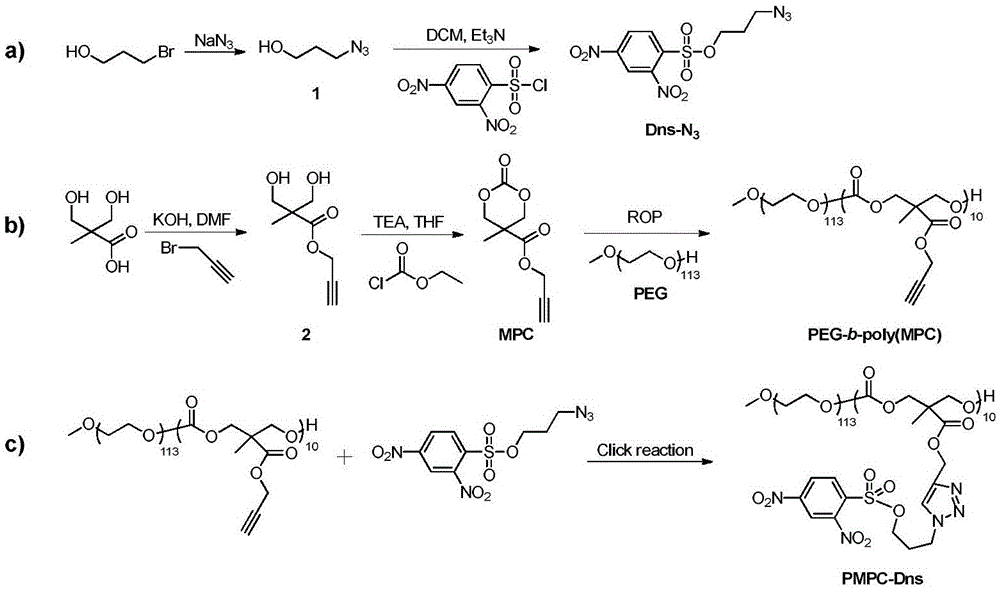
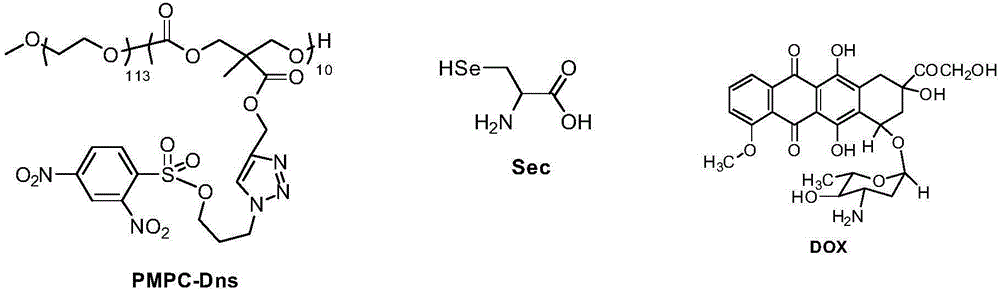
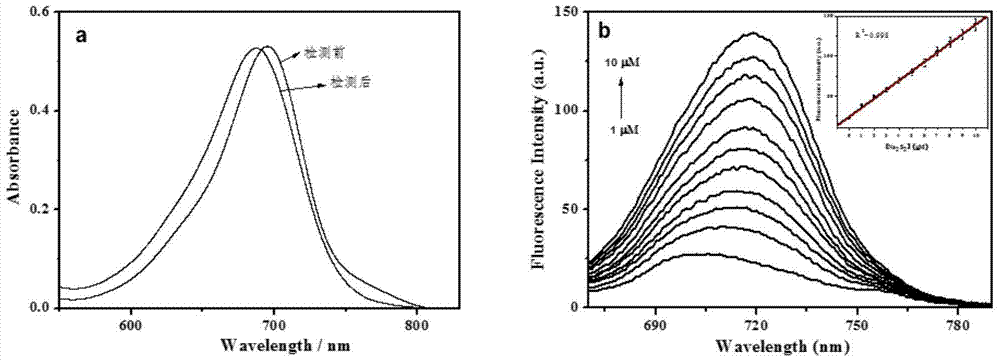
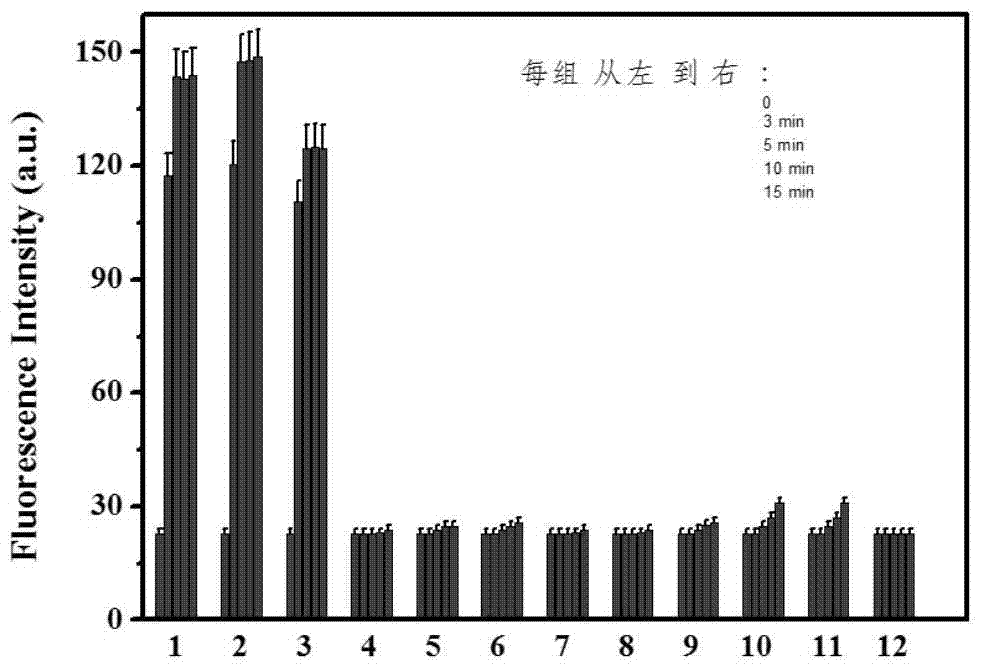
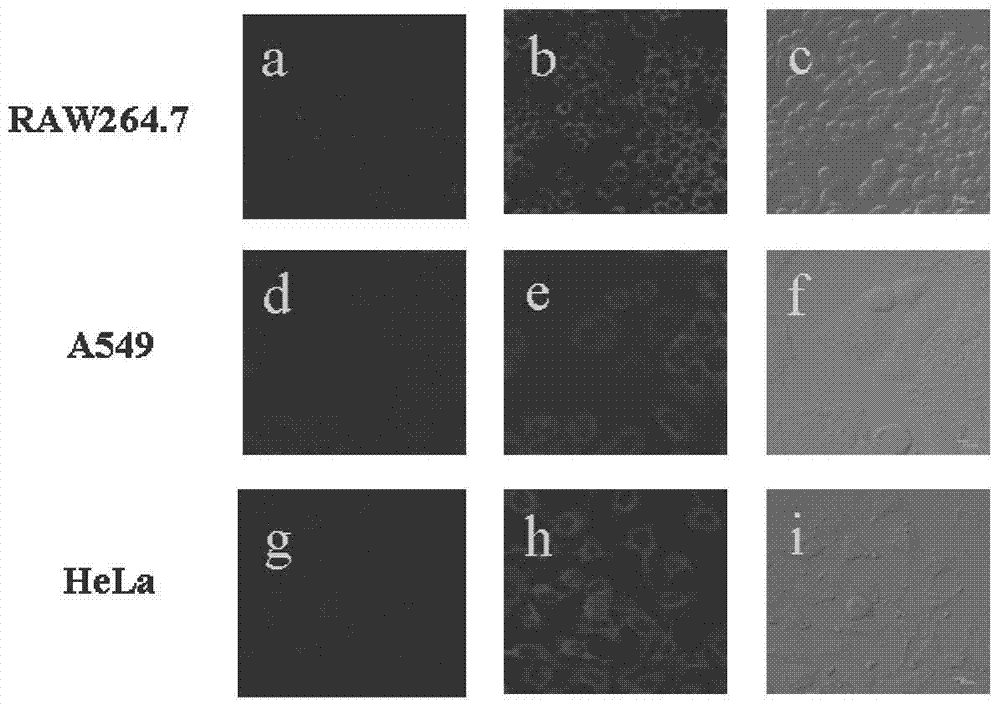
The invention reasonably designs and prepares a polycarbonate polymer, namely, PMPC-Dns, modified through 2,4-dinitrobenzene sulfonyl so as to detect selenocysteine in living bodies. The compound can wrap fluorescent medicine doxorubicin (DOX) and selectively respond to Sec and a selenol compound without being interfered with by biological mercaptan, amine and alcohol. Formation of micelle, responses of Sec, cytotoxicity of probes and medicine release of the Sec responses are researched. The PMPC-Dns probes can be applied to imaging of endogenous Sec in cervical cancer tissue and hela cells under the physiological conditions, and the PMPC-Dns probes can conduct imaging and release medicine wrapped in the probes at the same time. The work opens up a road for understanding the effects of Sec in physiology and pathology systems and tumor xenograft model systems, and a method is provided for controlled releases of hydrophobicity molecules in target molecules in the biomedicine application.
Owner:HUNAN UNIV
Small molecule photosensitizers for photodynamic therapy
The invention relates to small photosensitizers, their process of preparation and uses of the compounds in optical imaging and photodynamic therapy. The invention provides a compound of formula (I), a derivative or a salt thereof Wherein R1 is selected from the group consisting of amines, anilines, phenols, thiophenols, selenols and aryl groups; R2 and R3 independently are H or halogen; R4 is selected from the group consisting of H, nitre and cyano: and R5 and R6 independently are either absent or oxygen or methyl.
Owner:THE UNIV COURT OF THE UNIV OF EDINBURGH
A kind of preparation method of selenomethionine
ActiveCN106220539BThe synthesis process is simpleMild reaction conditionsOrganic chemistryHydrazine compoundMethyl carbonate
The invention discloses a preparation method for selenomethionine, which includes the steps of: (a) performing a reaction to L-methionine and dimethyl carbonate to remove a methylthio group and meanwhile form a ring to generate L-[alpha]-amino-[gamma]-butyrolactone, and adding hydrobromic acid to carry out a reaction to obtain L-[alpha]-amino-[gamma]-butyrolactone hydrobromate; (b) performing a reaction to selenium with hydrazine hydrate to generate sodium diselenide, adding a methylation reagent, dimethyl carbonate, to carry out a reaction to generate dimethyl diselenide, and reducing the dimethyl diselenide with sodium borohydride to obtain sodium methylselenide; and (c) performing a heating reflux reaction to the L-[alpha]-amino-[gamma]-butyrolactone hydrobromate and sodium methyl selenol to obtain L-selenomethionine sodium salt, and regulating the pH of the reaction liquid to 5-6 with acetic acid, and dehydrating the reaction liquid to obtain the target product L-selenomethionine. The preparation method has high product yield and simple synthetic process, is prepared from easy-to-obtain raw materials, has mild reaction conditions and is easy to carry out industrially.
Owner:SICHUAN SINYIML BIOTECH CO LTD
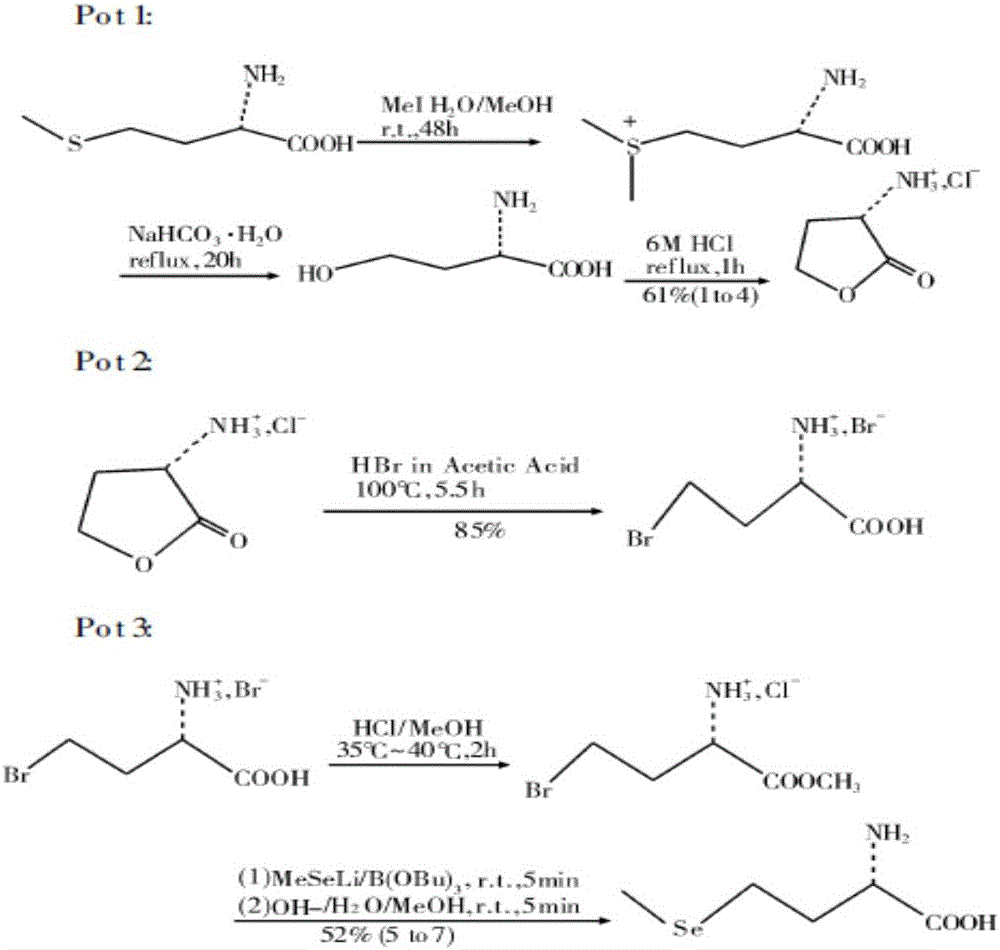
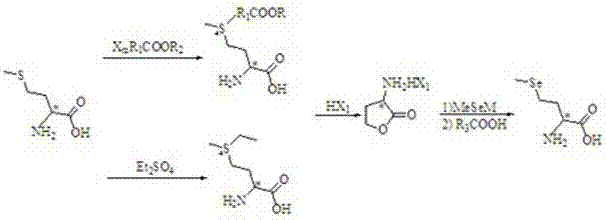
Preparation method of high-optical-purity D- or L-selenomethionine
The invention discloses a preparation method of high-optical-purity D- or L-selenomethionine. The preparation method comprises the following steps: by taking D- or L-methionine as initial raw materials and diethyl sulfate or halogenated alkyl acid or derived esters as alkylation reagents, generating sulfonium salt, desulfurizing and closing ring under acidic conditions, to produce alpha-amino-gama-butyrolactone halate, having addition reaction to alpha-amino-gama-butyrolactone halate with methyl selenol salt and opening ring, acidifying with organic acid, and recrystallizing to obtain D- or L-selenomethionine. The chemical purity of the product is more than or equal to 99%, and the high optical purity is more than or equal to 99%. The preparation method is low-cost and easily available in raw materials, simple in steps and easy to operate, simple in reaction conditions, and suitable for large-scale production.
Owner:安徽至善新材料有限公司
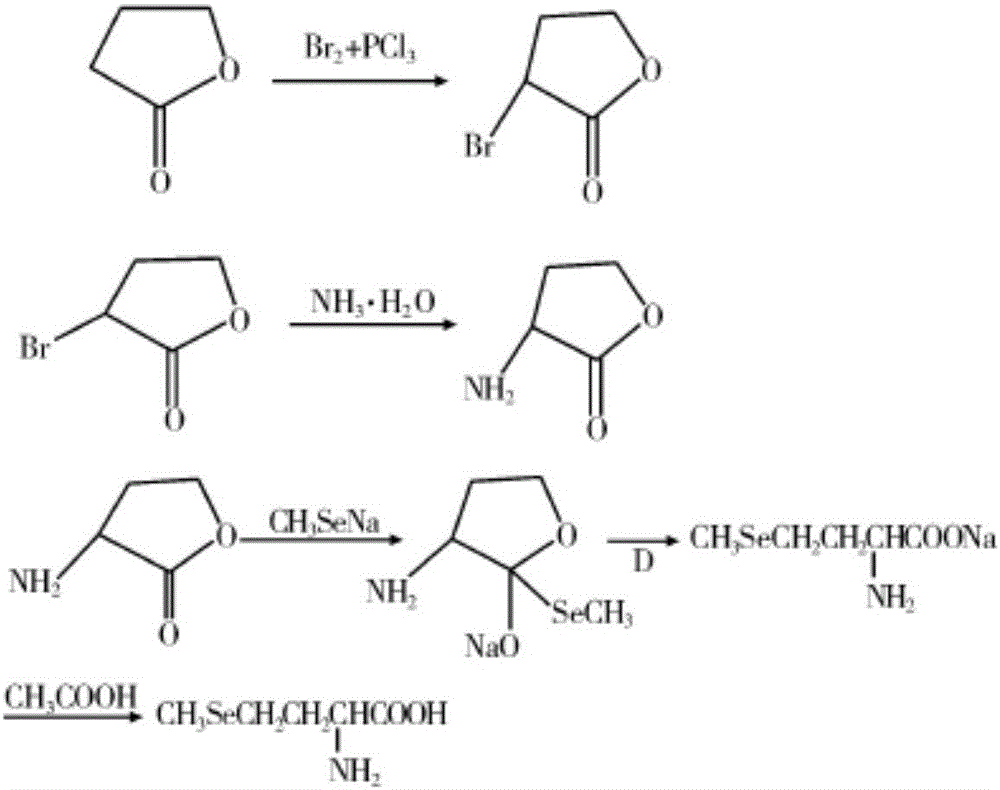
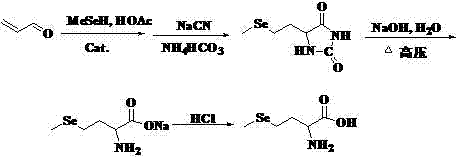
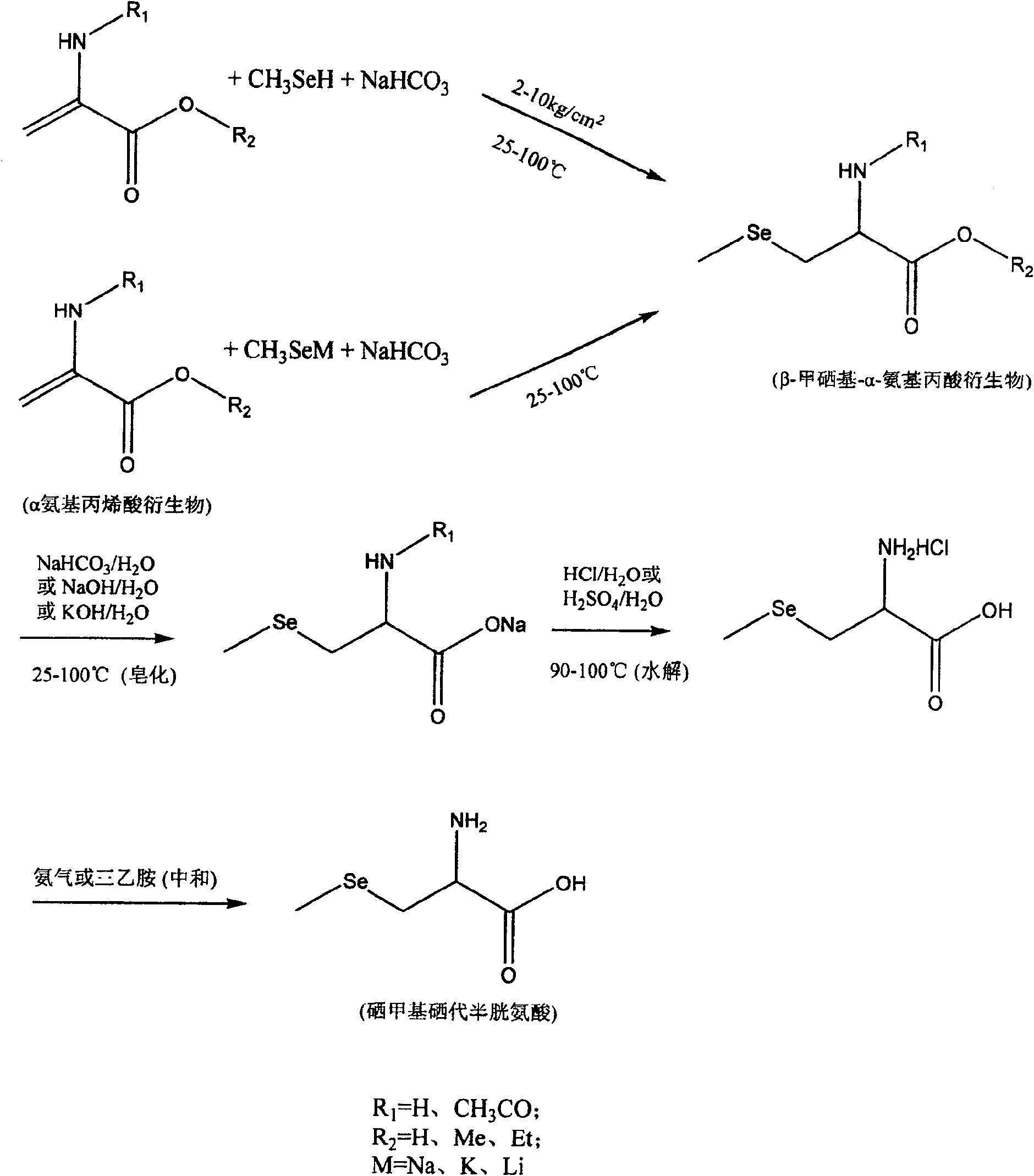
Method of preparing methylselenocysteine from alpha-amino acrylic acid derivative
The invention discloses a method for synthesizing selenomethylselenocysteine by using alpha aminoacrylic acid derivatives. First, the addition reaction of methylselenol or methylselenol salt solution with α-aminoacrylic acid derivatives generates β-methylselenyl-α-alanine derivatives, and then derivatizes β-methylselenyl-α-alanine The ester compound in the product is hydrolyzed and saponified by sodium bicarbonate, sodium hydroxide or potassium hydroxide, acidified with hydrochloric acid or sulfuric acid to obtain its carboxylic acid compound, and then the N-acyl group is hydrolyzed with hydrochloric acid or sulfuric acid to obtain β-methyl selenium Base-α-alanine hydrochloride or sulfate, and finally neutralized with ammonia or triethylamine to obtain selenomethylselenocysteine. The method has the advantages of simple synthetic route, high yield, environmental protection and low cost, and is suitable for large-scale industrial production.
Owner:江西川奇药业有限公司
Lysine compounds and their use in site- and chemoselective modification of peptides and proteins
InactiveUS8729009B2Fast and flexible and economicalEasy to synthesizeCarbamic acid derivatives preparationPeptide/protein ingredientsSelenolADAMTS Proteins
The present invention concerns new thiolysine and selenolysine compounds that can be used as building blocks for peptides and proteins, providing ligation handles for site- and chemoselective modification of said peptides and proteins. In particular, the invention provides. In particular, the invention provides (the use of) the compounds 5-thiolysine (also referred to as δ-thiolysine); 4-thiolysine (also referred to as γ-thiolysine); 5-selenolysine (also referred to as δ-selenolysine) and 4-selenolysine (also referred to as γ-selenolysine). The positioning of the thiol or selenol group at the respective carbon atom allows for a very efficient intramolecular transfer reaction to take place after conjugation with a selected ligand, and the thiol or selenol group may subsequently be removed using reported procedures, thereby restoring the native lysine structure, or be used as an additional conjugation handle. The methodology is fast and gives well-defined material.
Owner:NETHERLANDS CANCER INST
A kind of selenium-containing nonionic interfacial emulsifier and its preparation method and application
ActiveCN107998979BEasy to prepareSynthetic conditions are mildOrganic chemistryTransportation and packagingActive agentFatty acid
The invention discloses a selenium-containing nonionic interface emulsifier, a preparation method and application thereof, and belongs to the technical field of fine chemicals. The selenium-containing nonionic interfacial emulsifier is fatty acid benzyl selenium sulfoxide alkyl ester, and its chemical formula is C 6 h 5 CH 2 Se(O)(CH 2 ) m Se(O)(CH 2 ) m OC(O)(CH 2 ) n CH 3 , wherein m is an integer from 2 to 4, and n is an integer from 11 to 17. The preparation method of the selenium-containing nonionic interfacial emulsifier obtained in the present invention is simple, the synthesis condition is mild, there is no special equipment requirement, the energy consumption is low, and the raw material is easy to obtain, and the nonionic surfactant obtained by the method does not contain 1,4-dioxin ring; the hydrophilic group of the selenium-containing nonionic interfacial emulsifier is a selenium sulfoxide group that does not contain hydroxyl, polyoxyethylene and sugar groups; the selenium-containing nonionic interfacial emulsifier of the present invention does not possess obvious gas / liquid Active but with pronounced liquid / liquid interfacial activity.
Owner:JIANGNAN UNIV
Preparation of copper selenide nanoparticles
ActiveUS9196767B2Uniform sizeConfer solubilitySolid-state devicesSemiconductor/solid-state device manufacturingSelenolCopper selenide
A process for producing copper selenide nanoparticles by effecting conversion of a nanoparticle precursor composition comprising copper and selenide ions to the material of the copper selenide nanoparticles in the presence of a selenol compound. Copper selenide-containing films and CIGS semiconductor films produced using copper selenide as a fluxing agent are also disclosed.
Owner:NANOCO TECH LTD
Small tunable fluorophores for the detection and imaging of biomolecules
The invention relates to small, conjugatable, orthogonal and tunable fluorophores for imaging of small bioactive molecules. The invention further relates to processes for the preparation of the compounds, and uses of the compounds in therapeutic, diagnostic, surgery and analytical applications. The invention provides a compound of formula (I), a derivative or a salt thereof. Wherein X is selected from the group consisting of NH, O, S, SeR5R6, CR7R8; R1 is selected from the group consisting of amines, alcohols, thiols, thiophenols, selenols, selenophenols and aryl groups; R2 and R3 are independently H or a halogen; R4 tis either H, nitro or cyano; R5 is either absent or methyl or oxygen; R6 is either absent or methyl or oxygen; and R7 and R8 are independently selected from the group consisting of linear or cyclic alkyl groups containing halogen, amino, cyano or carboxylic ester substituents, and alkyl aryl groups.
Owner:THE UNIV COURT OF THE UNIV OF EDINBURGH
A class of d(π-a) based pyrazindole terminal receptors 2 Small molecule donor material and preparation method and application
ActiveCN110483555BImprove light absorption capacityLower HOMO levelOrganic chemistrySolid-state devicesHeterojunctionPyrazine
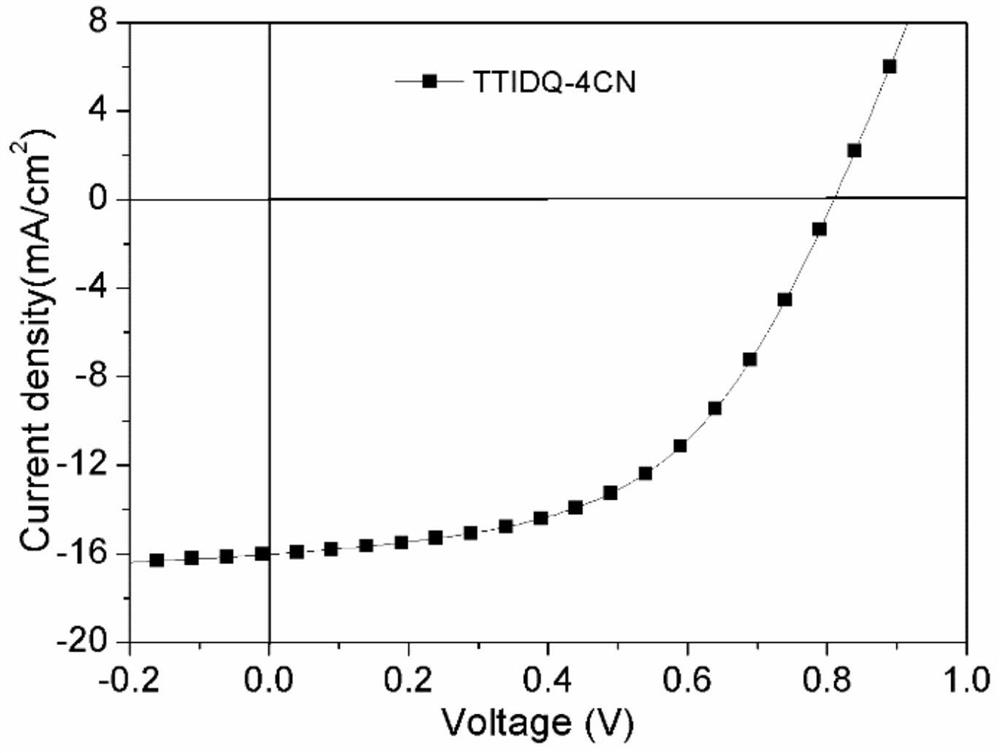
The invention belongs to the field of organic small molecule solar cells, in particular to a class of D(π-A) based on pyrazine indole terminal electron acceptors 2 Small molecule donor materials and their preparation methods and applications. where D(π‑A) 2 The electron-donating (D) unit of the type small molecule is 3,6-bis-(octylthio)thienothiophene (TT), and the π bridge unit is alkylated, oxyalkylated or sulfur-alkylated thiophene or selenophene , the electron-withdrawing (A) unit is a new type of pyrazine indole derivatives, and this type of small molecule donor material is used in solution-processed small molecule solar cells. by PC 71 BM is the acceptor, and the energy conversion efficiency of the bulk heterojunction solar cell reaches 7.31%. The invention realizes the high-efficiency energy conversion of the small molecule donor material based on the terminal acceptor unit of the pyrazine indole derivative in the small molecule solar cell.
Owner:CHANGZHOU UNIV
Fluorophore Derivatives Used for Determination of Polysulfurized Cysteine Fluoropyrrole Fluorophore and Its Application
ActiveCN105001250BReduce processing timeReduce testing costsGroup 3/13 element organic compoundsFluorescence/phosphorescencePhenacylBiotin
The invention relates to a fluorescent probe for detecting CysSnSSH (n is larger than one), in particular to a BODIPY derivative for measuring the CysSnSSH and application thereof. The BODIPY derivative is shown in the general formula I. In the general formula I, R1 (a detection group) is a sulfydryl benzoyl group or an aryl selenol benzoyl group or a tellurium phenol benzoyl group; R2 (a positioning functional group) is a sulfydryl benzoyl group, an aryl selenol benzoyl group, a tellurium phenol benzoyl group, a butyl triphenyl phosphonium group, an N-propyl morpholino group, a biotin group, a folic acid group, a glycosyl chain group or a C1-25 alkyl group; X represents H or halogen; Y represents N or C; Z represents N or O. In the presence of CysSnSSH, corresponding fluorescence intensity changes, and the compound like the CysSnSSH fluorescent probe can be used for detecting CysSnSSH, interference of external detection conditions can be greatly reduced, the sample treatment time is saved, and the detection precision is improved.
Owner:YANTAI INST OF COASTAL ZONE RES CHINESE ACAD OF SCI
A kind of method of synthesizing diselenide
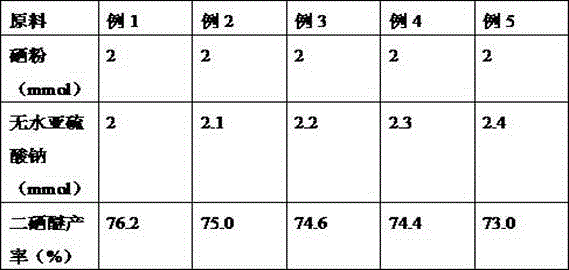
ActiveCN105130864BLess corrosiveSuitable for industrial productionOrganic chemistryChemical synthesisSulfite salt
A diselenide synthesizing method relates to the technical field of chemical synthesis. The method employs selenium powder as a selenylation reagent and anhydrous sodium sulfite as an additive, so that the method has many advantages. The method is free of any metal reagents. Because that selenium is a necessary microelement of living bodies and is metabolizable in human body, the method is ecological-friendly. In addition, the method is carried out under a neutral environment, so that the method is less in corrosion on devices and a reaction system is durable. The method does not generate a selenol intermediate which has stink smell, so that the method is suitable for industrial production, is low in cost and short in route, is mild in reaction conditions and is simple in reaction operations.
Owner:四川硒莱坞科技有限公司
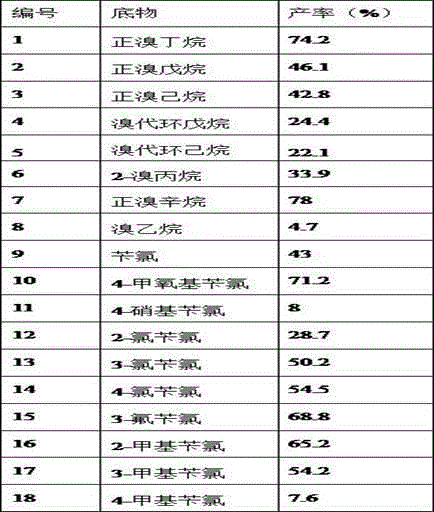
Method for synthesizing asymmetric selenide through selenol catalytic reaction
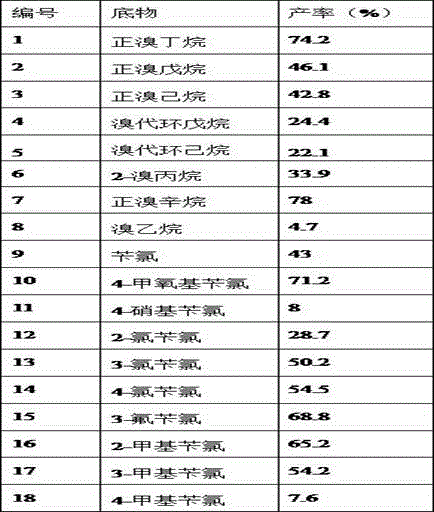
PendingCN113061107AHigh yieldAvoid harsh reaction conditionsOrganic chemistryHalohydrocarbonPhenylselenol
The invention discloses a method for synthesizing asymmetric selenide through selenol catalytic reaction. The method comprises the following steps: (1) sequentially adding anhydrous N, N-dimethylformamide, zinc chloride, phenylselenol, a strong base, halogenated hydrocarbon and a 4A molecular sieve into a reaction container; (2) stirring and reacting for 20-40 hours at normal temperature in an oxygen-free state; (3) filtering, and removing N, N-dimethylformamide and halogenated hydrocarbon to obtain a stock solution; and (4) diluting the stock solution obtained in the step (3) with diethyl ether, washing with water, drying, and distilling off the diethyl ether to obtain the asymmetric selenide. According to the method for synthesizing the asymmetric selenide provided by the invention, the low-proportion zinc chloride is adopted for reaction, so that the final product asymmetric selenide can be efficiently obtained with high yield; the method has the advantages that the use of cesium base, namely cesium hydroxide, which is not easy to commercialize or is very uneconomical is avoided, harsh reaction conditions are also avoided, the mild reaction conditions are adopted, the reaction time is greatly shortened, the atom economy is realized, and the yield of the finally obtained asymmetric selenide is relatively high.
Owner:无锡鸣鹭医药科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com