Lysine compounds and their use in site- and chemoselective modification of peptides and proteins
A compound, lysine technology, applied in the field of lysine compounds and their use in site-selective and chemoselective modification of peptides and proteins, can solve the problems of lack of available and flexible methods, involvement, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
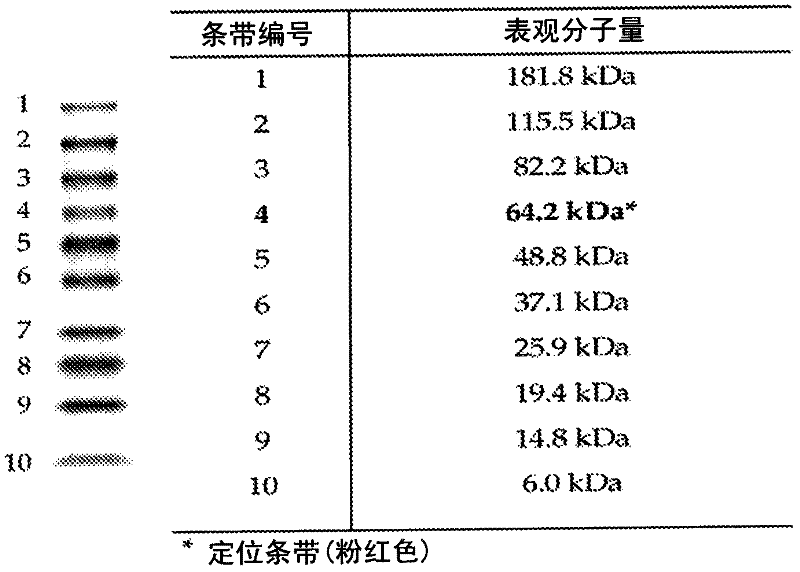
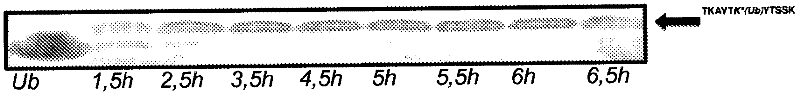
Image
Examples
Embodiment Construction
[0020] The first aspect of the present invention relates to the lysine compound represented by formula (Ia) or (Ib) or the ester, salt, solvate or hydrate of said lysine compound:
[0021]
[0022] Wherein -X- represents (i) sulfur or (ii) selenium; -P 1 and-P 2 independently represent (i) hydrogen or (ii) amine protecting group; -P 3 Represents (i) hydrogen; (ii) -X-R 6 The alkylthio, alkenylthio, alkylselenyl or alkenylselenyl shown, wherein -R 6 represents an optionally substituted branched or straight chain, aliphatic or cycloalkyl, heteroalkyl, alkenyl or heteroalkenyl moiety; (iii) a thiol or selenol protecting group; or (iv) -R 5 Part or (v) formula -C(=O)-R 5’ Part shown; -R 5 and -C(=O)-R 5’ represents the residue of a covalently bound ligand, preferably selected from peptides, lipids, carbohydrates, polymers, organic substances and inorganic substances; and -R 7 represents (i) hydrogen or (ii) optionally substituted branched or branched, aliphatic or cycloa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com