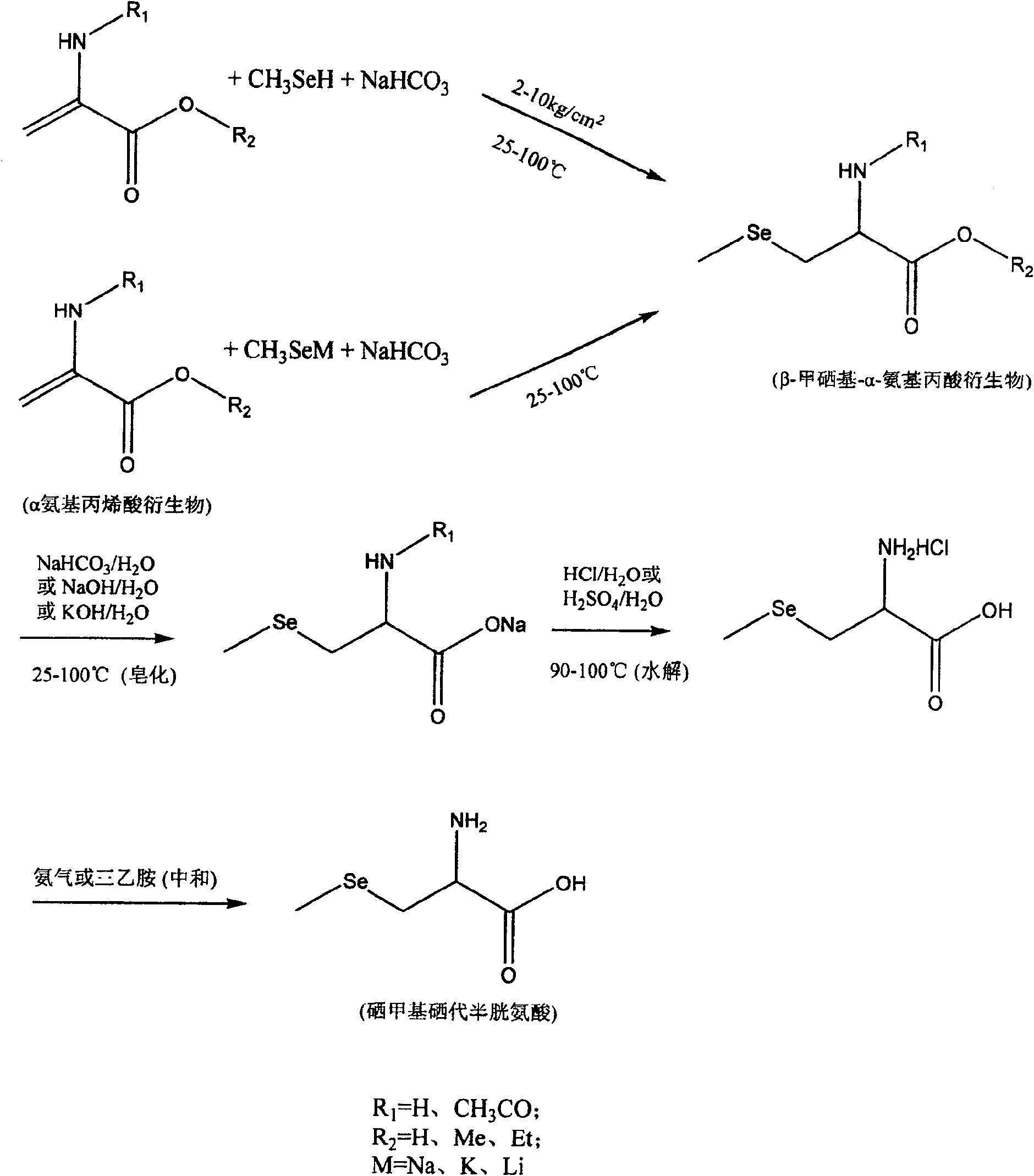
Method of preparing methylselenocysteine from alpha-amino acrylic acid derivative
A technology of α-aminoacrylic acid and selenocysteine, which is applied in the field of amino acid drug preparation, can solve the problems of high production cost, complex process route, and low yield, and achieve the effects of low cost, simple process, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Preparation of selenomethylselenocysteine using N-acetyl-α-aminoacrylic acid:
[0048] Dissolve 39 grams of N-acetyl-α-aminoacrylic acid in 300 ml of tetrahydrofuran, add 400 ml of water, add solid sodium bicarbonate to saturation, then add 40 grams of solid sodium bicarbonate, then pass through methylselenol at 25 ° C, and seal Keep 2kg / cm 2 Pressure, stirring reaction for 48 hours until N-acetyl-α-aminoacrylic acid reaction is complete (thin-layer chromatography detection); After removing the solid by filtration, use (1+1) hydrochloric acid to neutralize to a pH of about 1, and then use an equal volume of acetic acid Extract with ethyl ester, combine the organic phases and dry with anhydrous sodium sulfate, evaporate the solvent to dryness under reduced pressure to obtain a solid, dissolve it in methanol, remove the insoluble matter by filtration, place the methanol solution, and precipitate a white solid, recrystallize from methanol to obtain N-acetyl - 58 grams o...
Embodiment 2
[0051] Preparation of selenomethylselenocysteine using N-acetyl-α-aminoacrylic acid:
[0052] Dissolve 39 grams of N-acetyl-α-aminoacrylic acid in 300 ml of tetrahydrofuran, add 400 ml of water, add solid sodium bicarbonate to saturation, and then add 40 grams of solid sodium bicarbonate. Methylselenol was introduced at 65°C, and kept sealed at 2kg / cm 2 pressure, stirred and reacted for 40 hours until the reaction of N-acetyl-α-aminoacrylic acid was complete (detected by thin-layer chromatography). After the solid was removed by filtration, it was neutralized with (1+1) hydrochloric acid to a pH of about 1, then extracted with an equal volume of ethyl acetate, the organic phases were combined and dried with anhydrous sodium sulfate, and the solvent was evaporated to dryness under reduced pressure to obtain a solid , dissolved in methanol, filtered to remove insoluble matter, the methanol solution was placed, and a white solid was precipitated, recrystallized from methanol t...
Embodiment 3
[0055] Preparation of selenomethylselenocysteine using N-acetyl-α-aminoacrylic acid:
[0056] Dissolve 39 grams of N-acetyl-α-aminoacrylic acid in 300 ml of tetrahydrofuran, add 400 ml of water, add solid sodium bicarbonate to saturation, and then add 40 grams of solid sodium bicarbonate. Methylselenol was introduced at 100°C, and kept sealed at 10kg / cm 2 pressure, stirring and reacting for 30 hours until the N-acetyl-α-aminoacrylic acid was completely reacted (detected by thin-layer chromatography). After the solid was removed by filtration, it was neutralized with (1+1) hydrochloric acid to a pH of about 1, then extracted with an equal volume of ethyl acetate, the organic phases were combined and dried with anhydrous sodium sulfate, and the solvent was evaporated to dryness under reduced pressure to obtain a solid , dissolved in methanol, filtered to remove insoluble matter, the methanol solution was placed, and a white solid was precipitated, recrystallized from methanol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com