Polymer nano hydrogel and preparation method thereof
A nano-hydrogel and polymer technology, applied in the field of nano-hydrogel, can solve problems such as application limitations, achieve good biocompatibility, rapid release, and improve the effect of curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
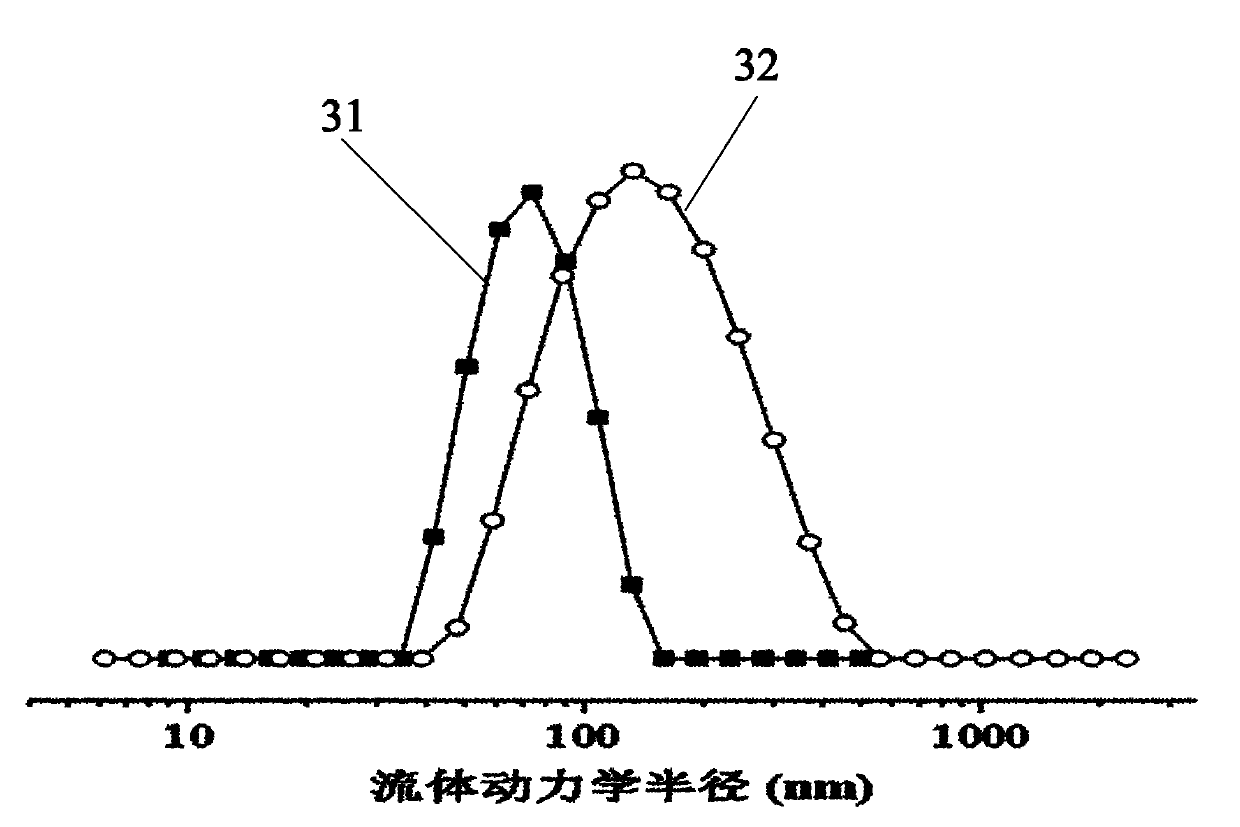
Image
Examples
preparation example Construction
[0040] The present invention also provides a method for preparing the polymer nanohydrogel described in the above technical scheme, comprising the following steps:
[0041] Dissolve the amino-terminated hydrophilic polymer, γ-benzyl-L-glutamate-N-endocarboxylic anhydride, and γ-2-chloroethyl-L-glutamate-N-endocarboxylic anhydride in In the water solvent, the stirring reaction obtains the first intermediate product, the γ-benzyl-L-glutamic acid ester-N-internal carboxylic acid anhydride and the γ-2-chloroethyl-L-glutamic acid ester-N- The ratio of the total number of moles of internal carboxylic acid anhydride to the number of moles of the amino-terminated hydrophilic polymer is 10 to 1000:1, and the γ-benzyl-L-glutamic acid ester-N-internal carboxylic acid anhydride and the The molar ratio of γ-2-chloroethyl-L-glutamate-N-internal carboxylic acid anhydride is 1:0.1~10, and the amino-terminated hydrophilic polymer has the structure of formula (III) or formula (IV) :
[0042] ...
Embodiment 1~5
[0074] Preparation of Example 1-5 terminal aminated polyethylene glycol monomethyl ether
[0075] Prepare end-aminated polyethylene glycol monomethyl ether according to the following method and the amount of raw materials shown in Table 1: After 10 g of polyethylene glycol monomethyl ether and 100 mL of toluene are azeotroped to remove water, dissolve in 100 mL of anhydrous dichloromethane After cooling to 0°C, add triethylamine, dropwise add methanesulfonyl chloride, react for 2h, return to 25°C and continue to stir for 24h, filter after the reaction, settle the filtrate with ether, filter, wash, 25°C Vacuum drying for 24 hours to obtain polyethylene glycol monomethyl ether methanesulfonate;
[0076] Mix 5g of polyethylene glycol monomethyl ether methanesulfonate with 5g of ammonium chloride, dissolve in 250mL of ammonia water, and react at 25°C for 72h. After the reaction, extract the reaction product with dichloromethane, and use a mass fraction of 4% Wash with an aqueous ...
Embodiment 6~10
[0082] The preparation of embodiment 6~10 terminal aminated polyethylene glycols
[0083] Prepare aminated polyethylene glycol according to the following method and the amount of raw materials shown in Table 3: After azeotroping 5 g of polyethylene glycol with 100 mL of toluene to remove water, dissolve it in 100 mL of anhydrous dichloromethane, and cool to 0 °C Finally, add triethylamine, dropwise add methanesulfonyl chloride, react for 2h, return to 25°C and continue to stir for 24h, filter after the reaction, settle the filtrate with ether, filter, wash, and vacuum dry at 25°C for 24h to obtain methyl Polyethylene glycol sulfonate;
[0084] Mix 5g of polyethylene glycol methanesulfonate with 5g of ammonium chloride, dissolve in 250mL of ammonia water, and react at 25°C for 72h. After the reaction, extract the reaction product with dichloromethane, and use chlorinated Wash with sodium aqueous solution, and then dry with anhydrous sodium sulfate. After fully drying, filter o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| radius | aaaaa | aaaaa |
| radius | aaaaa | aaaaa |
| hydrodynamic radius | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com