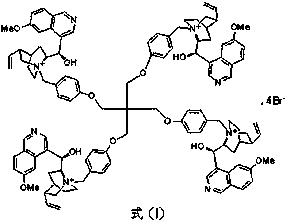
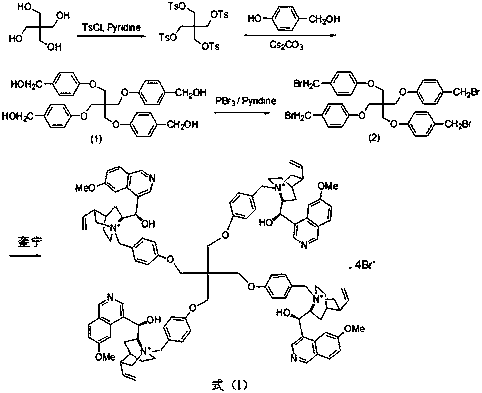
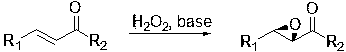Pentaerythritol-immobilized quinine catalyst as well as preparation method and application thereof
A technology for pentaerythritol and pentaerythritol benzene sulfonate is applied in the field of pentaerythritol-supported quinine catalyst and preparation thereof, can solve the problems of slow reaction speed, reduced catalytic activity and stereoselectivity, etc., and achieves high yield and simple synthesis route , the effect of constant catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The preparation of the quinine catalyst supported by pentaerythritol:
[0023] Add 2.04g (10.7mmol) of p-toluenesulfonyl chloride into a 250mL reaction flask, add 13mL of dry pyridine, stir at room temperature until all the solids are dissolved, cool the system to about 5°C in an ice bath, and add 0.34g (2.5 mmol) monopentaerythritol, stirred at room temperature for 8h. Add 50mL of water to the system, react for 2h, filter with suction, wash the solid with water, dissolve in dichloromethane, and precipitate with methanol to obtain 1.45g of pentaerythritol sulfonate in the form of white powder, with a yield of 78%. Mp: 148.5-150°C.
[0024] With 752mg (1mmol) pentaerythritol sulfonate, 546mg (4.4mmol) p-hydroxybenzyl alcohol and 1.43g (4.4mmol) Cs 2 CO 3 Add to 10mL dry DMF, react at 60°C for 24h, filter, evaporate the solvent under reduced pressure, add 10mL CH 2 Cl 2 Dissolved, washed with saturated brine, dried, concentrated, and purified by column chromatography...
Embodiment 2
[0028] (1R,2S )-1-phenyl-2-benzoyloxirane preparation:
[0029] Add 500mg (2.4mmol) styryl benzophenone, 53mg (0.024mmol) pentaerythritol-supported quinine and 0.03mL Span-20 into 7mL diisopropyl ether, stir, and add 30% H 2 o 2 (2.7mL, 24mmol) and 50% KOH (0.27mL, 2.4mmol) aqueous solution, stirred vigorously, reacted at room temperature for 4h, concentrated under reduced pressure to half of the original volume, added diethyl ether, precipitated, filtered, washed with diethyl ether, and dried in vacuo , Recover the catalyst, the recovery rate is 97%. The filtrate was washed with water, dried, filtered, concentrated under reduced pressure, and purified by column chromatography to obtain (1R,2S )-1-phenyl-2-benzoyloxirane. Yield: 95%, ee: 99.5%.
Embodiment 3
[0031] ( 1R,2S )-1-(2'-fluorophenyl)-2-benzoyloxirane preparation:
[0032] Replace 500mg (2.4mmol) styryl benzophenone with 542mg (2.4mmol) 2-fluorostyryl benzophenone, replace 0.03mL Span-20 with 0.03mL Tween-20, other operations are the same as in Example 2, get( 1R,2S )-1-(2’-fluorophenyl)-2-benzoyloxirane, yield 97%, ee: 99.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com